国际多中心,随机II期研究DC Bead与传统lipodol TACE与阿霉素 International multicentre, randomized phase II study of DC Bead uploaded with doxorubicin versus conventional Lipiodol TACE with doxorubicin 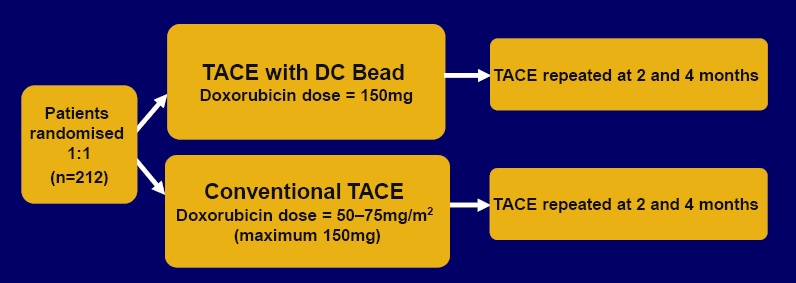
• Primary endpoint: tumor response at 6 months (EASL criteria by blinded independent review of MR images)
• Secondary endpoints included safety (toxicity according to SWOG)
SWOG癌症研究网络是由美国国家癌症研究所(NCI)资助的一个癌症临床试验小组
TACE with DC Bead vs conventional TACE: enrollment criteria
TACE with DC Bead vs conventional TACE: treatment-related adverse events *

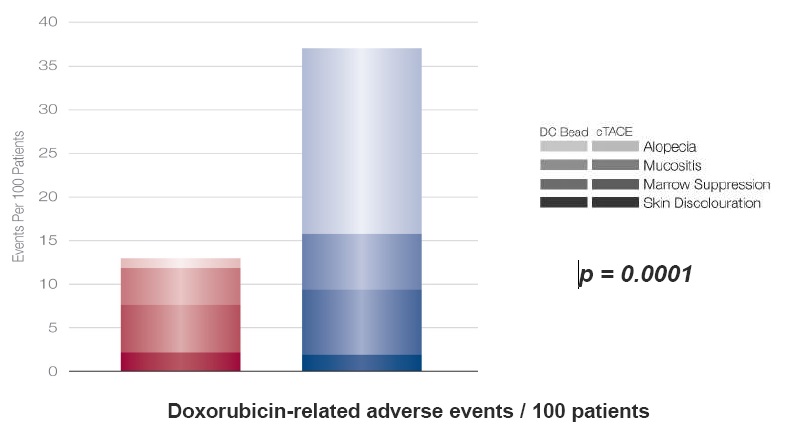
TACE with DC Bead vs conventional TACE: doxorubicin-related adverse events
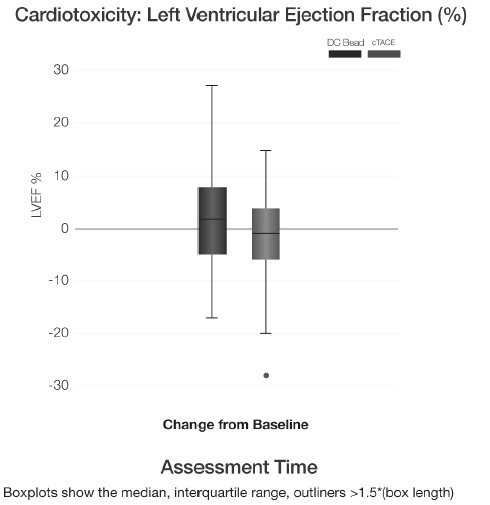
TACE with DC Bead vs conventional TACE: cardiac toxicity (LV ejection fraction)
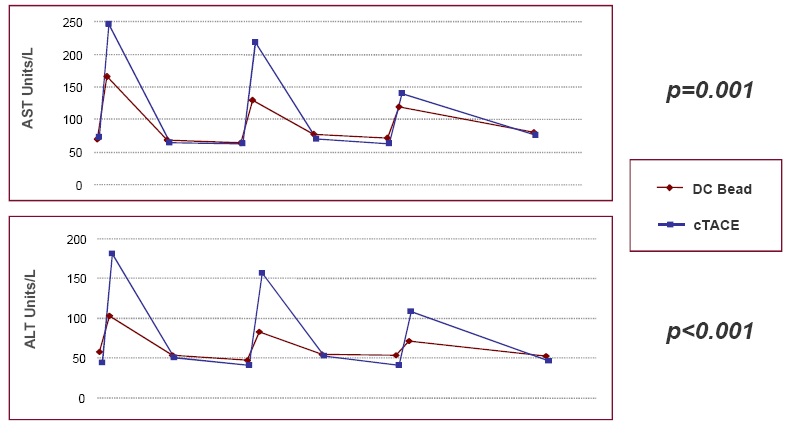
TACE with DC Bead vs conventional TACE: liver toxicity (ALT-AST levels)
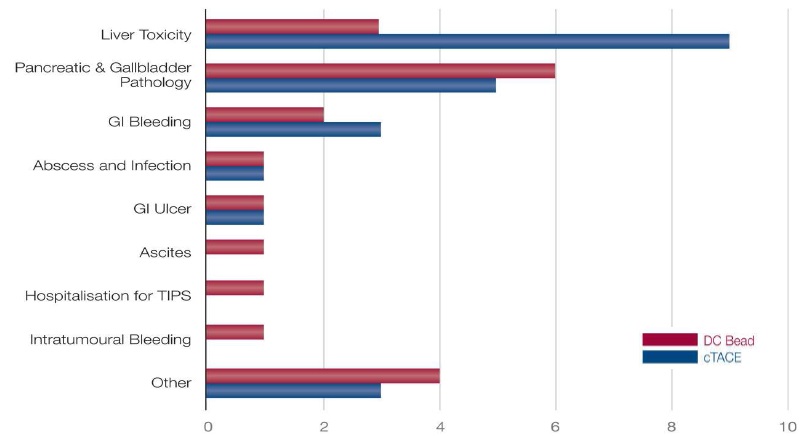
TACE with DC Bead vs conventional TACE: severe adverse events
TACE with DC Bead vs conventional TACE: tumor response at 6 months
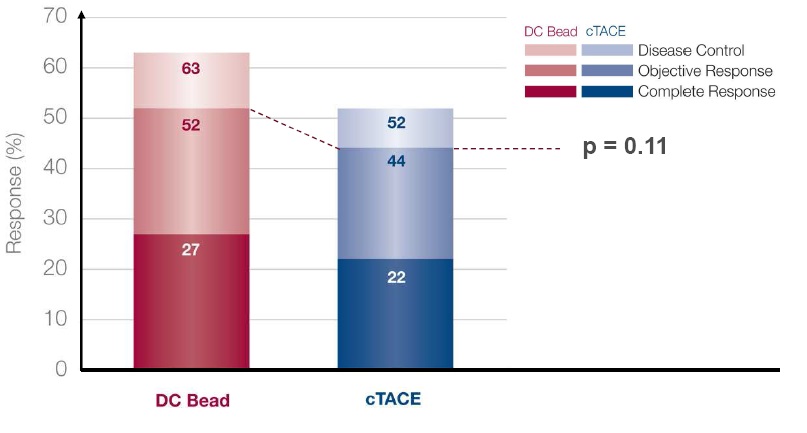
TACE with DC Bead vs conventional TACE: tumor response at 6 months
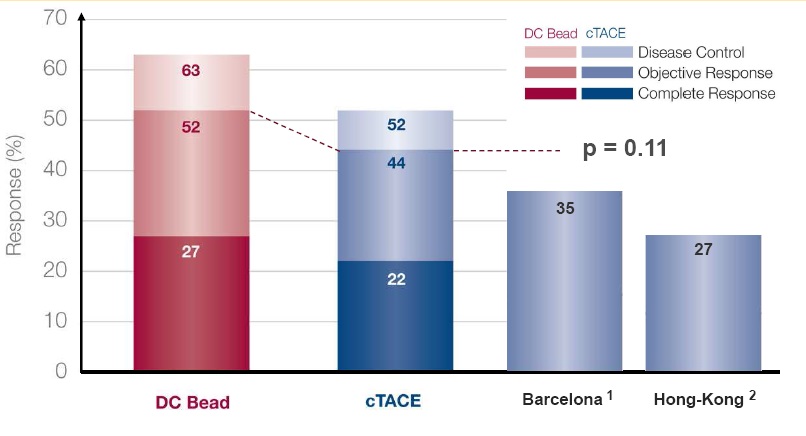
1. Llovet JM et al. Lancet 2002;359:1734–9
2. Lo CM et al. Hepatology 2002;35:1164–71
TACE with DC Bead vs conventional TACE: tumor response – subgroup analysis
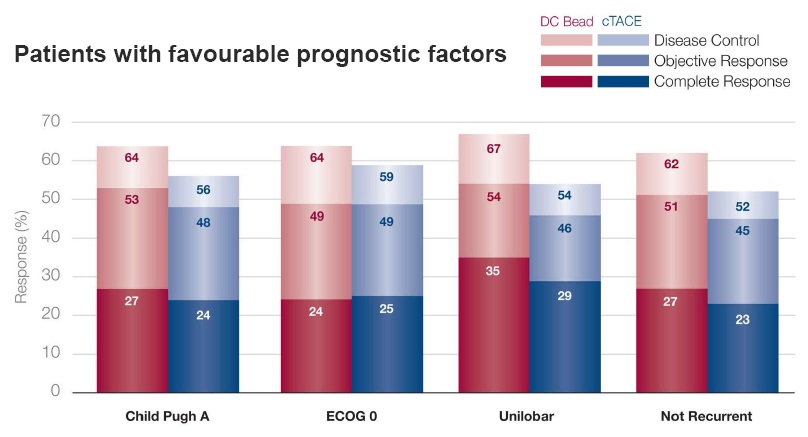
TACE with DC Bead vs conventional TACE: tumor response – subgroup analysis
|

