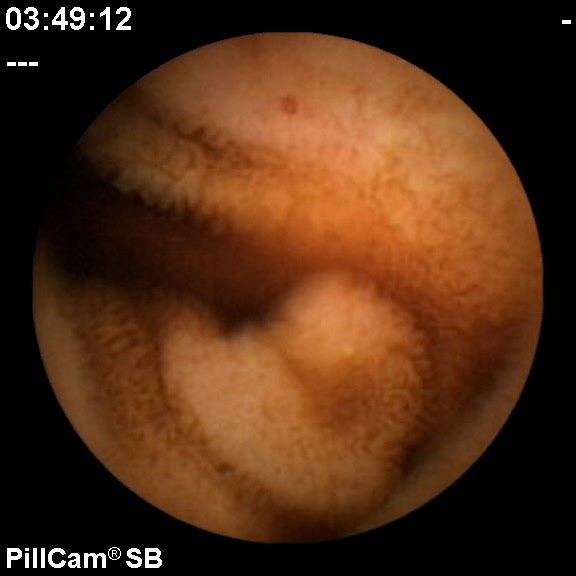
一旦获得详细的病史、体检以及常规的实验室检查。各种不同的特殊手段都可以应用于各种类型的潜血性和不明消化道出血,包括各种类型的内窥镜,常规X线检查,CT或CTA,核医学现象,血管造影甚至外科手术。有关胃肠道出血病人的最初的评估和处理都有文献综述[28]。并不在本栏目赘述。这里仅强调小肠不明出血中活跃出血的诊断和定位,在很少的情况下这种出血需要介入治疗扮演部分角色。
小肠出血精确定位的检查 1. 胃镜,12指肠镜和结肠镜:尽管上消化道出血和下消化道出血首先考虑内窥镜检查。但下消化道大出血急诊内窥镜可能由于结肠腔内大量粪便和血凝块限制对肠粘膜的观察,尽管冲洗可能改善这种情况。主要用于除外小肠出血。如果反复标准内窥镜检查都不能发现病变,进一步的评估取决于出血的活泼性。
 (2)新型双气囊小肠镜(Double-Balloon Enteroscope ,DBE):工作长度2500~2790mm,能使观察小肠的长度增加至150cm。可以远达回肠。也可以通过结肠逆行进入小肠,因为检查耗时,仅仅应用于其它检查未能发现整个小肠的时候。2000年首先由Yamamoto报告,2004年被批准在美国的应用。一项研究显示,双球囊肠镜可以发现出血源为74%(40%~80%)的病人[20~23]。但在亚洲和西方文献中DBE所发现的病是不一样的,在亚洲主要是溃疡或肿瘤[24,25],而在西方文献中则主要是血管增生不良性病变[26,27]。
  (3)探条式肠镜(Sonde enteroscopy):直径5mm,长3000mm,无活检工作通道,镜身纤细柔软。经鼻插入后可达空肠,其后随肠蠕动继续前进,6~7小时后可抵达回盲部(仅10%),到达回肠的成功率77%~84%;退镜时观察肠粘膜,由于头端方向不能自如控制,粘膜面观察不全面,因此诊断阳性率50%。有报告,482经其它方法没有检查出病变的病例,经此项检查确定80%为动静脉畸形[15]。检查主要用于出血间歇期或生命体征稳定的消化道出血,缺点是耗时[7~9],病人较为痛苦,并导致出血和穿孔的并发症,假阴性率较高。因此临床应用受到限制[10,11]。 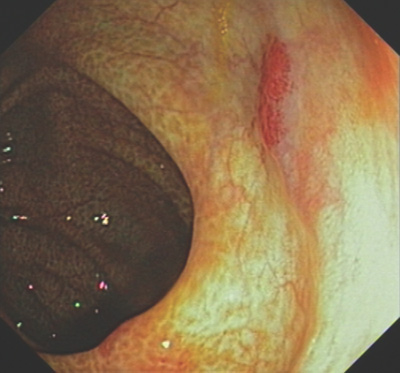 内窥镜下的 angiodysplasia
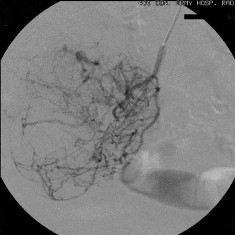
肿瘤可以表现为异常肿瘤血管,血管移位和肿瘤染色。有报告,血管扩张剂或溶栓,抗凝可提高动脉造影对出血的诊断率(32% vs 65%),这一措施是基于导管在血管内,易于对可能的出血进行控制。
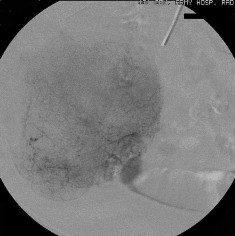
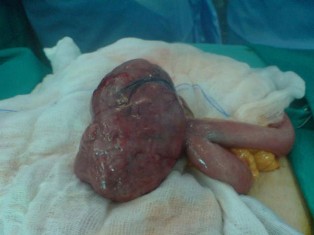
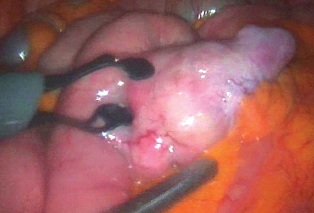
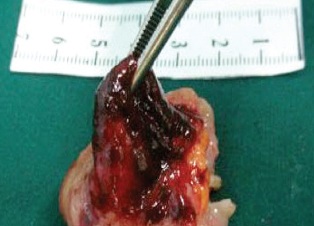
除去血管扩张和肿瘤,有些其它良性病变也表现为血管异常。下图为小肠憩室
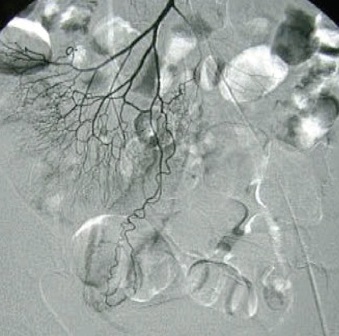 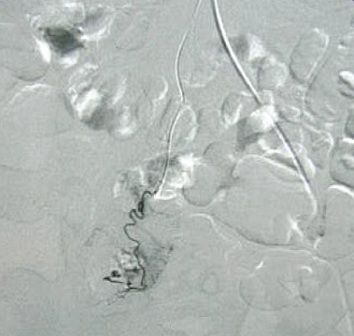   较次一级的检查是发现出血的大体位置(Low yield tests in identifying bleeding site) 1. 全消化道造影和小肠造影(粘膜病变较难发现)和小肠气钡造影( Small Bowel follow-through test , Enteroclysis. Double-contrast Barium Enema) :这是传统影像学检查小肠出血的三部曲。尽管全消化道钡剂造影在小肠出血诊断中广泛应用,但隐性出血的诊断率低。Rabe 和Gordon 等分别对215例和46例病人行全消化道钡餐检查,诊断率分别为5.6%和6.5%。消化道造影后短期内影响血管造影进行。小肠低张气钡造影也较广泛用于小肠出血的诊断,较全消化道造影可提高出血诊断率10%~15%。但仅对小肠肿瘤的诊断率高(95%的诊断率,显示小肠造影仍然是较好的初选检查),对粘膜病变和血管病变诊断率低,甚至不能发现绝大多数血管病变,导致假阴性。有报告43例小肠出血病人进行小肠气钡造影,仅13例有阳性发现,其中12例为小肠肿瘤,1例为Meckel‘s憩室。 2. CT血管造影[36]:可以经静脉注射,也可以进行动脉(肠系膜动脉和主动脉)注射造影剂后进行CT扫描。动脉注射的阳性率为72%(13/18),9例阳性结果7例证实为小肠出血,包括小肠血管增生不良及肿瘤各两例。 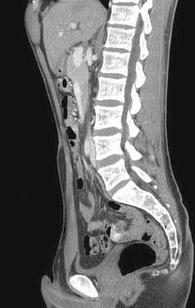 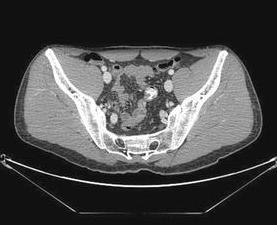 CT血管造影显示造影剂直接外溢至肠腔内,术后证实为Meckel‘s 憩室
Kuhle and Sheiman 建议螺旋CT发现急性下消化道出血需0.5ml/min,这是通过肠系膜上动脉造影所引证的[47]。而最新的报告显示,多探头螺旋CT用于急性大量下消化道出血的敏感性和特异性超过90%[48]
3. 标记红细胞同位素显像(ECT):属非创伤性检查,敏感性高,但需要活跃出血(较高的假阳性),不能精确定位,更不能提示出血原因,其发现影响不明消化道出血病人处理是有限的。尽管比肠系膜动脉造影的敏感性高,但后者可以更精确的定位和同时进行治疗。同位素扫描的主要适应症是不明来源的急、慢性消化道出血,最常用于血管造影前的检查。用鍀99标记的红细胞进行放射性核素扫描。被标记的红细胞可以在体内循环48小时。出血的部位可以通过γ相机记录,可以发现出血率低至0.05~0.1mm/min的消化道出血[49]。发现可疑的出血部位通常需要血管造影,内窥镜和外科手术加以证实。被证实的出血的精确率在26%~78%[1,50]。最敏感是在扫描的头2小时。如果内窥镜和CT检查阴性,核素扫描可以改善出血的整体发现率。 4. CT 肠造影(CT enterography)[35]:常规X线检查和内窥镜评价小肠受到小肠的长度,直径和小肠袢运动的影响。最新多探头CT扫描技术的发展,可以有更快速的扫描时间,和更高的空间分辨率,和多角度和平面评估小肠壁和肠腔。CT肠造影正常CT扫描一样,口服被稀释的钡剂和静脉注射造影剂后进行CT扫描,和标准CT扫描不一样,CT肠造影可以更多地发现小肠粘膜的改变。 5. MRI 肠造影(CT enterography):同上,只是口服的造影剂不一样。 6. 吞线实验:是消化道出血传统而古老的诊断方法,简易,实用和经济。 7. Meckel's憩室扫描[46]: 8. 剖腹探查:被迫对不知出血位置的开腹探查手术,对外科医生来说面对的是非常困难的情况。仅能发现较大的血管或肿瘤病变。在大多数情况下小肠血管病变导致的出血,在间歇期上台手术,即看不到也摸不到,盲目切除可能导致术后并发症。 目前并无经过验证的标准的小肠出血检查程序可供参考。隐匿性小肠出血在检查程序上较为从容,但对于急性小肠出血可供选择的方法主要是CT增强或选择性动脉造影,如果病人一般情况尚可,先进行核素扫描最好。 明显消化道出血的诊治策略(1): 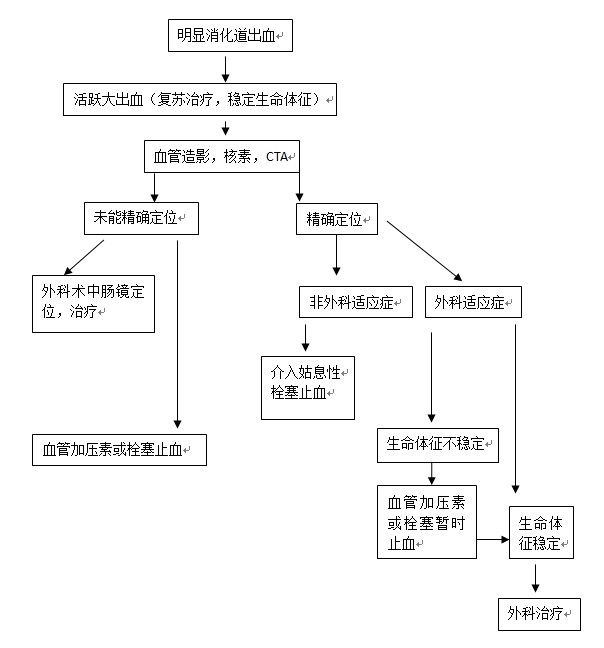 明显消化道出血的诊治策略(2):
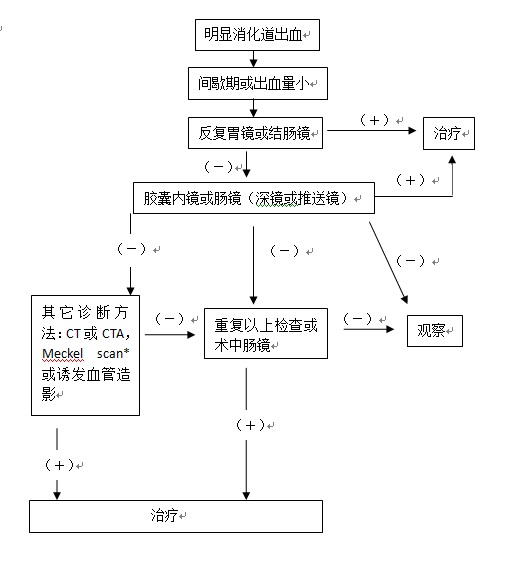 如果重复内镜检查没有发现病变,进一步的评价取决于出血的活跃性。活跃出血的病人应该进行核素扫描或血管造影。前者仅可以帮助确定出血的部位,但对明显消化道出血病人的处理的影响是有限的。肠系膜血管造影对于小肠出血的敏感度低于核素扫描,但出血定位更精确,更能够确定适合的治疗方案。其它的诊断方法如CT肠造影[31]或CTA[32],以及Meckel 憩室扫描对某些病人有帮助。 * 消化道憩室中异位黏膜显像:消化道憩室尤其是小肠Meckel憩室中异位胃黏膜的存在造成邻近黏膜的溃疡,有时可发生大出血。在这种情况下显示异位胃黏膜的存在对确定诊断有举足轻重的意义。根据99mTc-锝酸盐浓聚于胃黏膜的分泌细胞而该种细胞常伴随泌酸壁细胞存在的特点,在静脉注射上述示踪剂后30min扫描,可能发现异位泌酸黏膜,阳性率可达80%。 1. Hunter JM, Pezim ME. Limited value of technetium 99m-labeled red cell scintigraphy in localization of lower gastrointestinal bleeding. Am J Surg. 1990 May;159(5):504-6.
2. Romaozinho JM, Pontes JM, Lerias C, Ferreira M, Freitas D. Dieulafoy's lesion: management and long-term outcome. Endoscopy 2004; 36: 416-420 3. Sass DA, Chopra KB, Finkelstein SD Schauer PR. Jejunal gastrointestinal stromal tumor: a cause of obscure gastrointestinal bleeding. Arch Pathol Lab Med 2004; 128: 214-217 4. Bodner J, Chemelli A, Zelger B, Kafka R. Bleeding Meckel's diverticulum. J Am Coll Surg 2005; 200: 631 5. Rerksuppaphol S, Hutson JM, Oliver MR. Ranitidineenhanced 99mtechnetium pertechnetate imaging in children improves the sensitivity of identifying heterotopic gastric mucosa in Meckel’s diverticulum. Pediatr Surg Int 2004; 20: 323-325 6. Ming-Chen Ba, San-Hua Qing, Xiang-Cheng Huang, Ying Wen, Guo-Xin Li, Jiang Yu Diagnosis and treatment of small intestinal bleeding: Retrospective analysis of 76 casesWorld J Gastroenterol 2006 December 7; 12(45): 7371-7374 7. Keuchel M, Hagenmuller F. Small bowel endoscopy. Endoscopy 2005; 37: 122-132 8. Warneke RM, Walser E, Faruqi S, Jafri S, Bhutani MS, Raju GS. Cap-assisted endoclip placement for recurrent ulcer hemorrhage after repeatedly unsuccessful endoscopic treatment and angiographic embolization: case report. Gastrointest Endosc 2004; 60: 309-312 9. Nguyen NQ, Rayner CK, Schoeman MN. Push enteroscopy alters management in a majority of patients with obscure gastrointestinal bleeding. J Gastroenterol Hepatol 2005; 20: 716-721 10. Ell C, Remke S, May A, Helou L, Henrich R, Mayer G. The fi rst prospective controlled trial comparing wireless capsule endoscopy with push enteroscopy in chronic gastrointestinal bleeding. Endoscopy 2002; 34: 685-689 11. Hartmann D, Schmidt H, Bolz G, Schilling D, Kinzel F, Eickhoff A, Huschner W, Moller K, Jakobs R, Reitzig P, Weickert U, Gellert K, Schultz H, Guenther K, Hollerbuhl H, Schoenleben K, Schulz HJ, Riemann JF. A prospective two-center study comparing wireless capsule endoscopy with intraoperative enteroscopy in patients with obscure GI bleeding. Gastrointest Endosc 2005; 61: 826-832 12. Jones BH, Fleischer DE, Sharma VK, Heigh RI, Shiff AD, Hernandez JL, Leighton JA. Yield of repeat wireless video capsule endoscopy in patients with obscure gastrointestinal bleeding. Am J Gastroenterol 2005; 100: 1058-1064 13. Lewis BS, Swain P. Capsule endoscopy in the evaluation of patients with suspected small intestinal bleeding: Results of a pilot study. Gastrointest Endosc 2002; 56: 349-353 14. Martinez-Ares D, Gonzalez-Conde B, Yanez J, Estevez E, Arnal F, Lorenzo J, Diz-Lois MT, Vazquez-Iglesias JL. Jejunal leiomyosarcoma, a rare cause of obscure gastrointestinal bleeding diagnosed by wireless capsule endoscopy. Surg Endosc 2004; 18: 554-556 15. Lewis BS.Small intestinal bleeding.Gastroenterol Clin North Am. 2000 Mar;29(1):67-95, vi. Review. 16. M Mylonaki, A Fritscher-Ravens, P Swain. Wireless capsule endoscopy: a comparison with push enteroscopy in patients with gastroscopy and colonoscopy negative gastrointestinal bleeding. Gut 2003;52:1122-1126 17. Stuart L Triester MD, Jonathan A Leighton MD, Grigoris I Leontiadis MD, David E Fleischer MD, Amy K Hara MD, Russell I Heigh MD, Arthur D Shiff MD and Virender K Sharma MD A Meta-Analysis of the Yield of Capsule Endoscopy Compared to Other Diagnostic Modalities in Patients with Obscure Gastrointestinal Bleeding Am J Gastroenterol 100: 2407-2418; 18. Foutch PG (1993) Angiodysplasia of the gastrointestinal tract. Am J Gastroenterol 88:807-818 19. Lau WY, Yuen WK, Chu KW, Poon GP, Li AK (1992) Obscure bleeding in the gastrointestinal tract originating in the small intestine. Surg Gynecol Obstet 174:119-124 20. May A, Nachbar L, Wardak A, Yamamoto H, Ell C. Double-balloon enteroscopy: preliminary experience in patients with obscure gastrointestinal bleeding or chronic abdominal pain. Endoscopy 2003; 35: 985-991 21. Yamamoto H, Kita H, Sunada K, et al.Clinical outcomes of double-balloon endoscopy for the diagnosis and treatment of small-intestinal diseases.Clin Gastroenterol Hepatol,2004, 2: 1010-1016. 22.May A, Nachbar L, Ell C. Double-balloon enteroscopy (push-and-pull enteroscopy) of the small bowel: feasibility and diagnostic and therapeutic yield in patients with suspected small bowel disease. Gastrointest Endosc 2005; 62: 62-70
23. Mehdizadeh S, Ross A, Gerson L et al. What is the learning curve associated with double-balloon enteroscopy? Technical details and early experience in 6 U.S. tertiary care centers. Gastrointest Endosc 2006;64:740–750.
24. Manabe N, Tanaka S, Fukumoto A, Nakao M, Kamino D, Chayama K. Double-balloon enteroscopy in patients with GI bleeding of obscure origin. Gastrointest Endosc 2006; 64: 135-140
25. Nakamura M, Niwa Y, Ohmiya N, Miyahara R, Ohashi A, Itoh A, Hirooka Y, Goto H. Preliminary comparison of capsule endoscopy and double-balloon enteroscopy in patients with suspected small-bowel bleeding. Endoscopy 2006; 38: 59-66
26. Di Caro S, May A, Heine DG, Fini L, Landi B, Petruzziello L, Cellier C, Mulder CJ, Costamagna G, Ell C, Gasbarrini A. The European experience with double-balloon enteroscopy: indications, methodology, safety, and clinical impact. Gastrointest Endosc 2005; 62: 545-550
27. Heine GD, Hadithi M, Groenen MJ, Kuipers EJ, Jacobs MA, Mulder CJ. Double-balloon enteroscopy: indications, diagnostic yield, and complications in a series of 275 patients with suspected small-bowel disease. Endoscopy 2006; 38: 42-48 28. Rockey, D. C. Gastrointestinal bleeding. Gastroenterol. Clin. North Am. 34, 581–588 (2005).
29. Jensen, D. M. Current diagnosis and treatment of severe obscure GI hemorrhage. Gastrointest. Endosc. 58, 256–266 (2003). 30. Leighton, J. A. et al. Obscure gastrointestinal bleeding. Gastrointest. Endosc. 58, 650–655 (2003). 31. Huprich, J. E. et al. Obscure gastrointestinal bleeding: evaluation with 64-section multiphase CT enterography--initial experience. Radiology 246, 562–571 (2008). 32. Saperas, E. et al. Capsule endoscopy versus computed tomographic or standard angiography for the diagnosis of obscure gastrointestinal bleeding. Am. J. Gastroenterol. 102, 731–737 (2007). 33. de Leusse, A. et al. Capsule endoscopy or push enteroscopy for first-line exploration of obscure gastrointestinal bleeding? Gastroenterology 132, 855–862; quiz 1164–1165 (2007). 34. Bresci, G., Parisi, G., Bertoni, M., Tumino, E. & Capria, A. The role of video capsule endoscopy for evaluating obscure gastrointestinal bleeding: usefulness of early use. J. Gastroenterol. 40, 256–259 (2005). 35. Huprich, J. E. et al. Obscure gastrointestinal bleeding: evaluation with 64-section multiphase CT enterography--initial experience. Radiology 246, 562–571 (2008). 36. Saperas, E. et al. Capsule endoscopy versus computed tomographic or standard angiography for the diagnosis of obscure gastrointestinal bleeding. Am. J. Gastroenterol. 102, 731–737 (2007). 37. Triester, S. L. et al. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am. J. Gastroenterol. 100, 2407–2418 (2005). 38. Pennazio, M. et al. Outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: report of 100 consecutive cases. Gastroenterology 126, 643–653 (2004). 39. May, A., Wardak, A., Nachbar, L., Remke, S. & Ell, C. Influence of patient selection on the outcome of capsule endoscopy in patients with chronic gastrointestinal bleeding. J. Clin. Gastroenterol. 39, 684–688 (2005). 40. Viazis, N. et al. Impact of capsule endoscopy in obscure small-bowel bleeding: defining strict diagnostic criteria for a favorable outcome. Gastrointest. Endosc. 62, 717–722 (2005). 41. Rastogi, A., Schoen, R. E. & Slivka, A. Diagnostic yield and clinical outcomes of capsule endoscopy. Gastrointest. Endosc. 60, 959–964 (2004). 42. Lai, L. H. et al. Long-term follow-up of patients with obscure gastrointestinal bleeding after negative capsule endoscopy. Am. J. Gastroenterol. 101, 1224–1228 (2006). 43. Zuckerman, G. R., Prakash, C., Askin, M. P. & Lewis, B. S. AGA technical review on the evaluation and management of occult and obscure gastrointestinal bleeding. Gastroenterology 118, 201–221 (2000). 44. Lin, S. & Rockey, D. C. Obscure gastrointestinal bleeding. Gastroenterol. Clin. North Am. 34, 679–698 (2005). 45. Laine L, Sahota A, Shah A. Does capsule endoscopy improve outcomes in obscure gastrointestinal bleeding? Randomized trial versus dedicated small bowel radiography. Gastroenterology. 2010 May;138(5):1673-1680.e1; quiz e11-2. Epub 2010 Feb 2.
47. Kuhle WG, Sheiman RG. Detection of active colonic hemorrhage with use of helical CT: findings in a swine model. Radiology. 2003 Sep;228(3):743-52.
48. Yoon W, Jeong YY, Shin SS, Lim HS, Song SG, Jang NG, Kim JK, Kang HK. Acute massive gastrointestinal bleeding: detection and localization with arterial phase multi-detector row helical CT. Radiology. 2006 Apr;239(1):160-7. Epub 2006 Feb 16. 49. Gupta S, Luna E, Kingsley S, Prince M, Herrera N. Detection of gastrointestinal bleeding by radionuclide scintigraphy. Am J Gastroenterol. 1984 Jan;79(1):26-31. 50. Diehl SJ, Ko HS, Dominguez E, Kaare Tesdal I, Kähler G, Böhm C, Düber C. Negative endoscopy and MSCT findings in patients with acute lower gastrointestinal hemorrhage. Value of (99m)Tc erythrocyte scintigraphy. [Article in German] Radiologe. 2007 Jan;47(1):64-70. (责任编辑:Mr.Editor) |

|