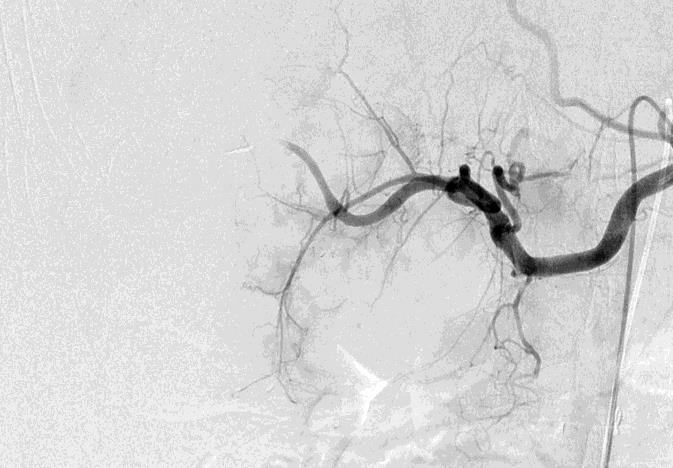
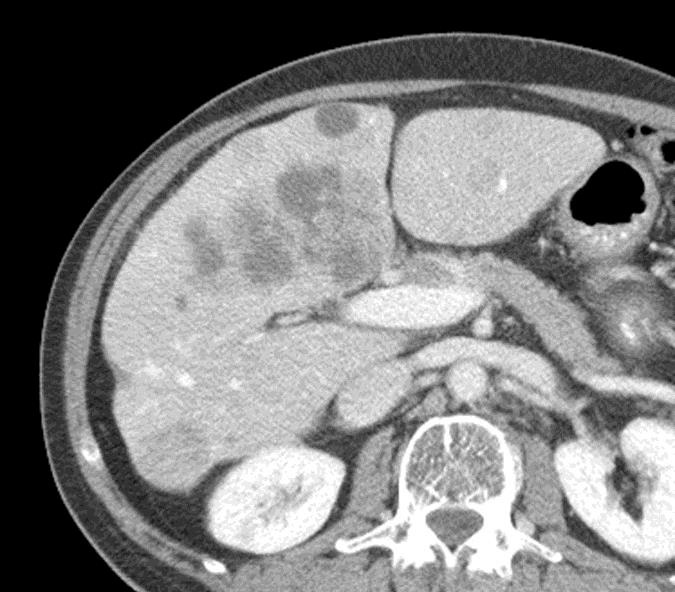
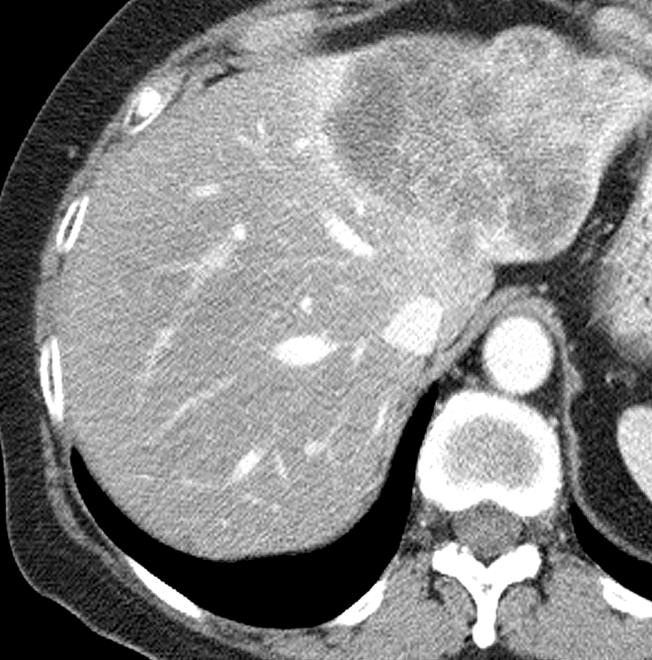
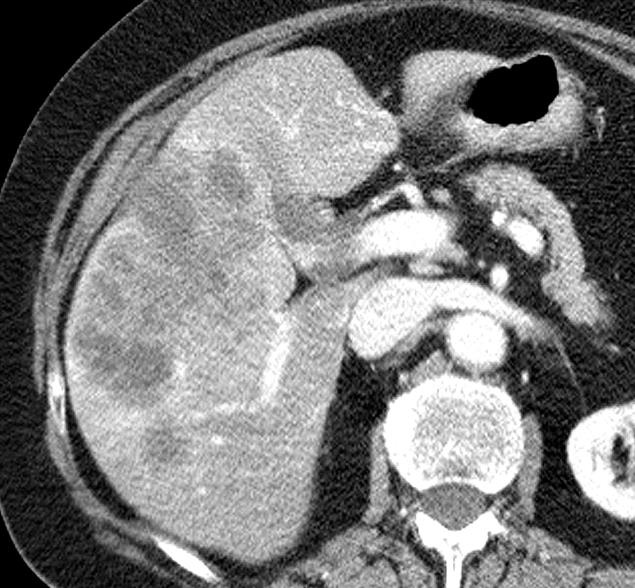
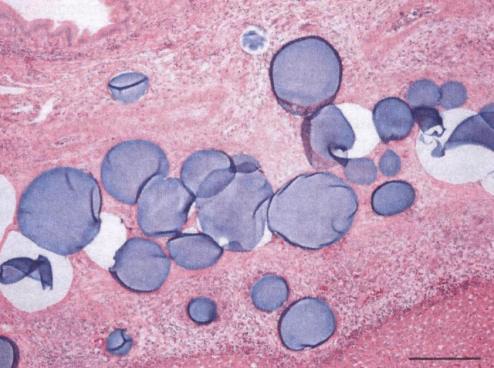
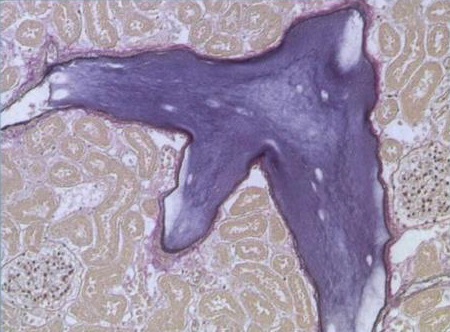
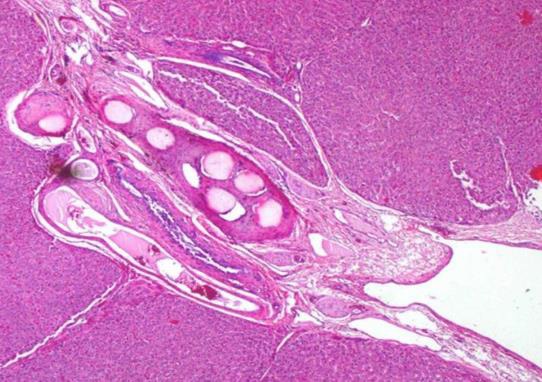
1. 载药微球-顺铂
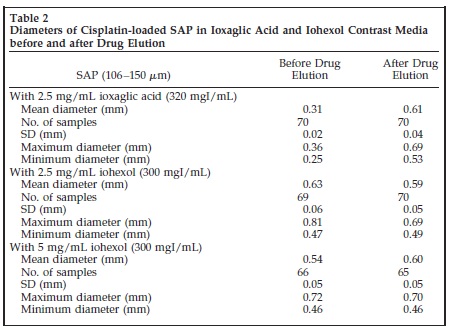
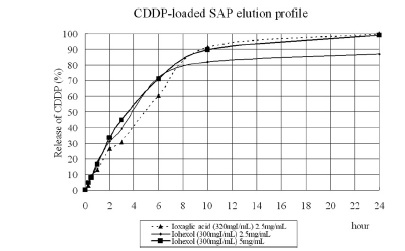
ioxaglic and iohexol contrast the elution profile was similar with cisplatin fractions of 15%,40%,70% and 95% at 1,3,6 and 24 hours
respectively
• Conducted a preclinical study in vitro
• In vivo in patients with CRC measuring oxaliplatin concentration in target lesion (by biopsy) and plasma
• Highest of drug tested was 400mg oxaliplatin/50mg HepaSphere
• Working dose : 50mg oxaliplatin + 1 vial of Hepa 50-100 for 15min
3. 伊立替康- HepaSphere 文献
C= Cisplatin; D=Doxorubicin; M=Mitomycin C; FU=5-FU; INF=Interferon; Mel=Melphalan; Gem= Gemcitabin, I=Irinotecan
* RECIST 这些历史上TACE的文献比较历史上HAI文献,局部的ORR并没有得到改善。在二线治疗(SL)中与静脉化疗没有比较研究。有人指出“结直肠癌肝转移TACE的研究并没有成熟”【4】。
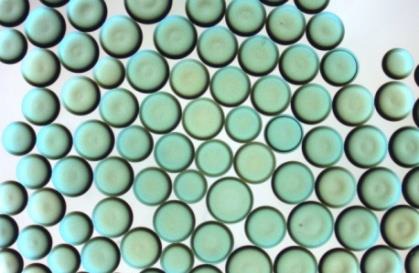
DEB-TACE 伊立替康(Irinotecan-eluting HepaSphere)药物洗脱微球® 完全坏死=化学消融(chemoablation)
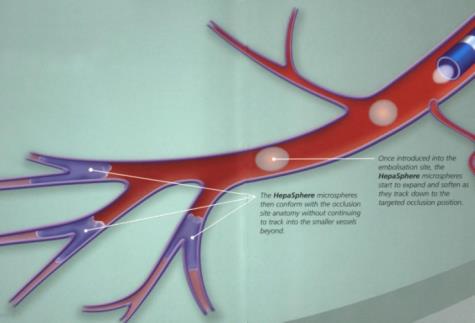
Chemoembolization of hepatic metastases using drug eluting microparticles has the potential of Chemoablation. Which microparticles do we have? 我们有哪些微粒?
Evidence from Literature【5】
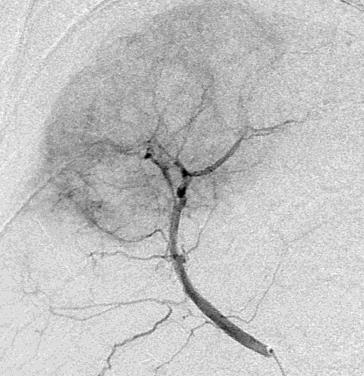
拯救化疗,前瞻性研究, 29例病人71次TACE 治疗计划
endpoint:stopflow
局部反应
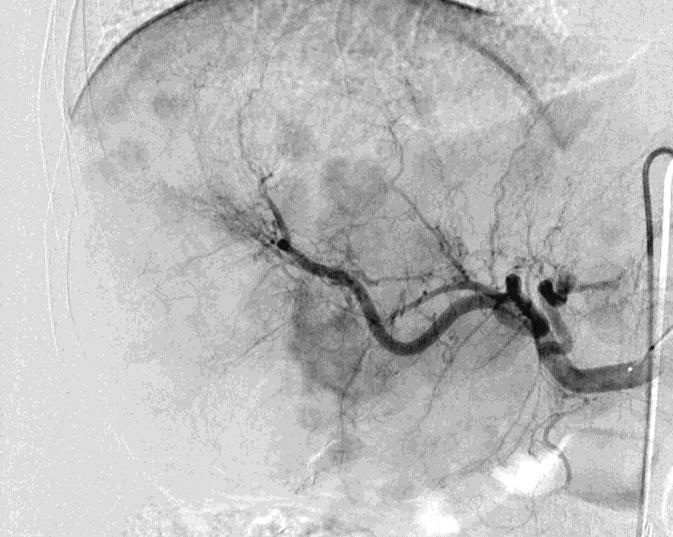
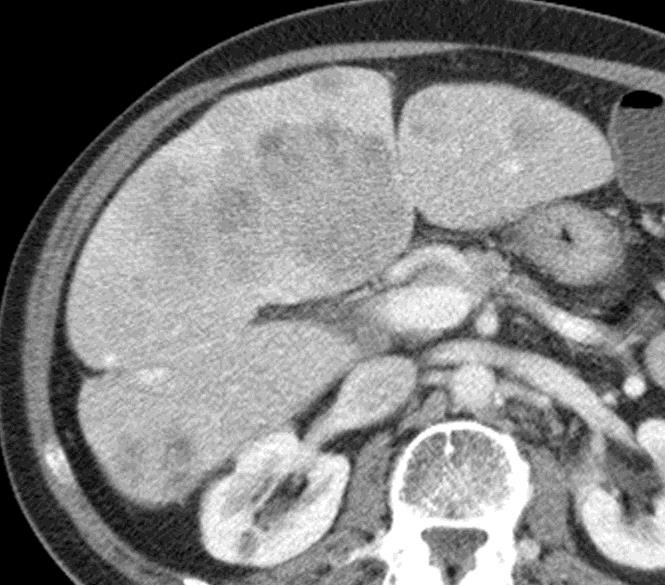
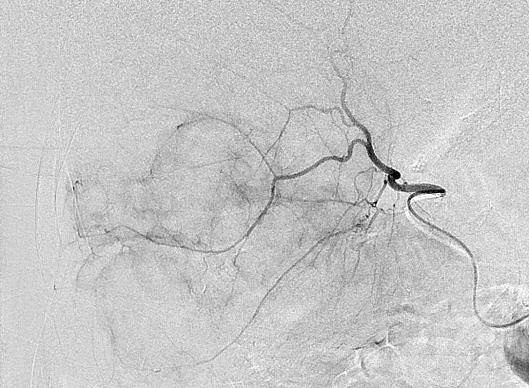
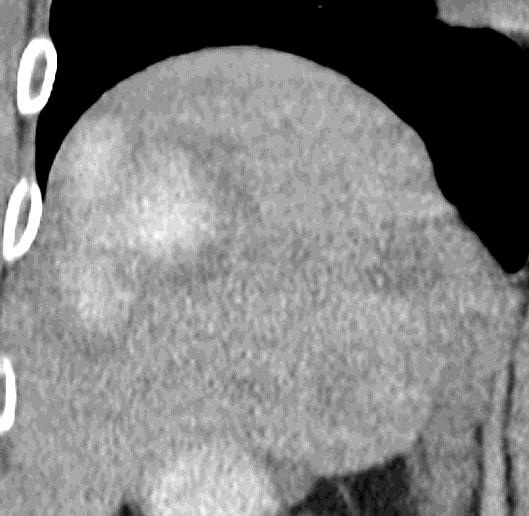
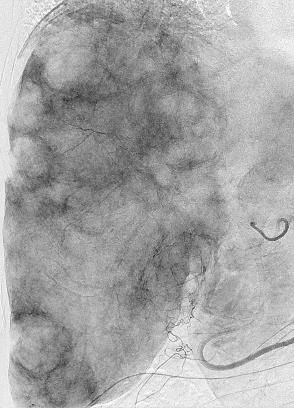
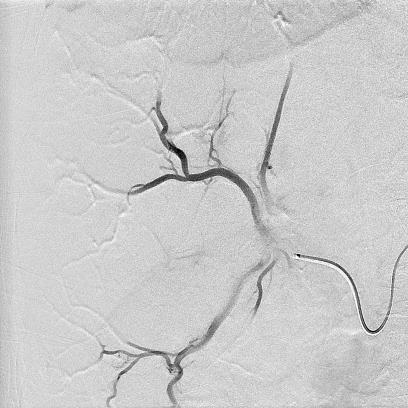
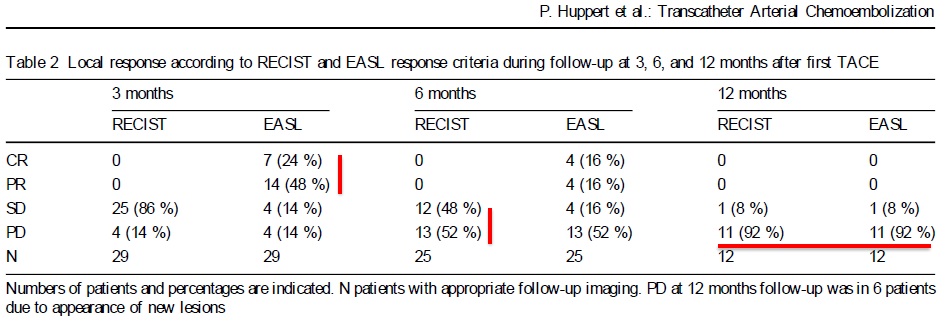
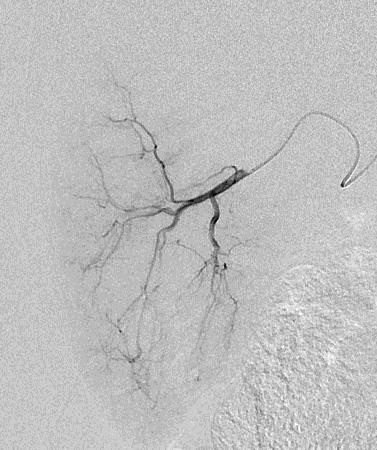
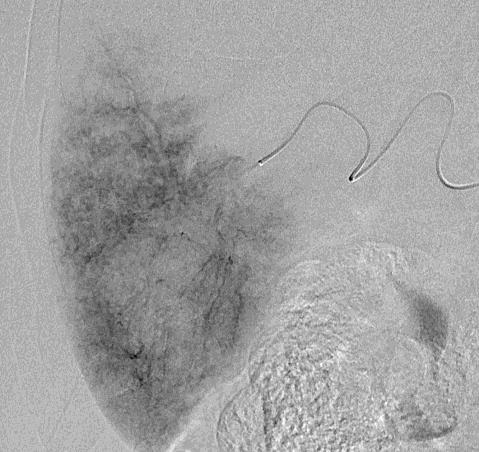
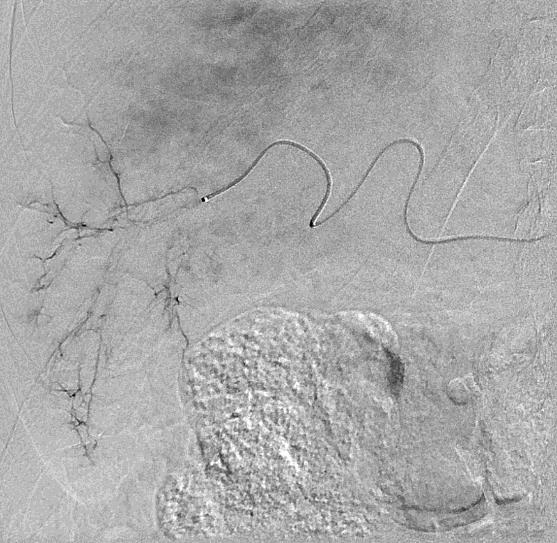
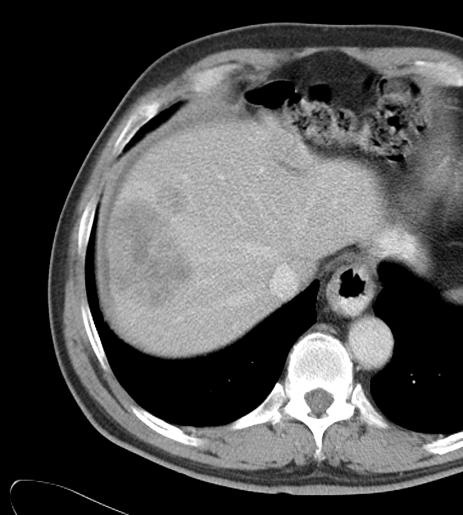
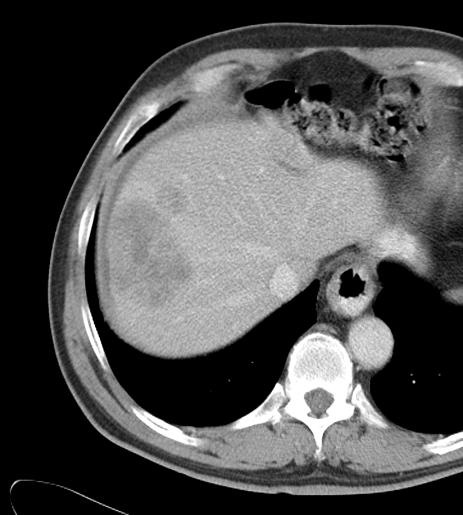
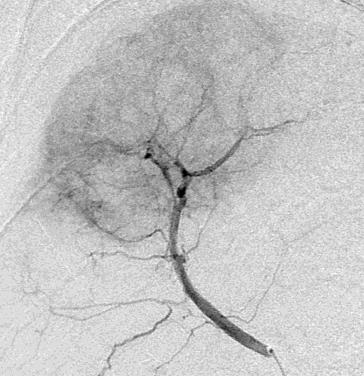
Can we predict local response? Uptake of CM/MP(造影剂/微球的吸收)
Uptake of contrast day +1
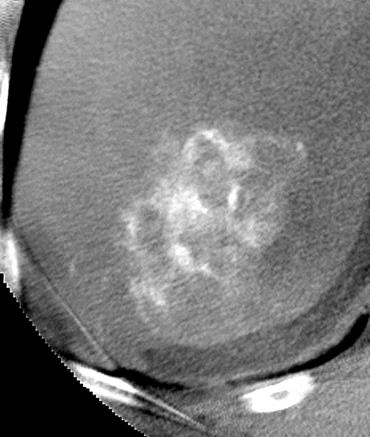
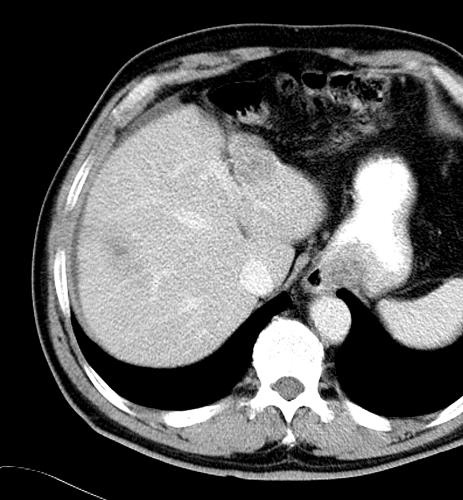
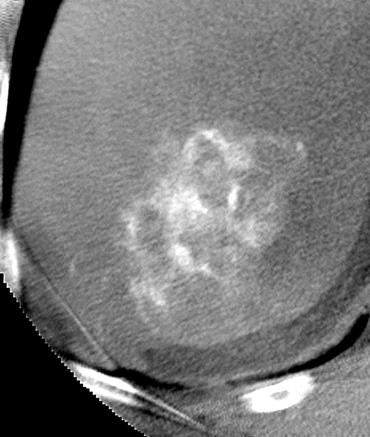
Uptake grade 3 (HepaSphere 50mg, Irinotecan 200mg)
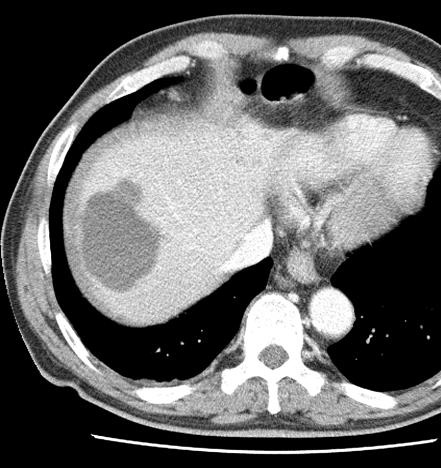
Uptake grade 0-1
Uptake predicts local response(HepaSphere™ 100 mg,Irinotecan 400 mg)
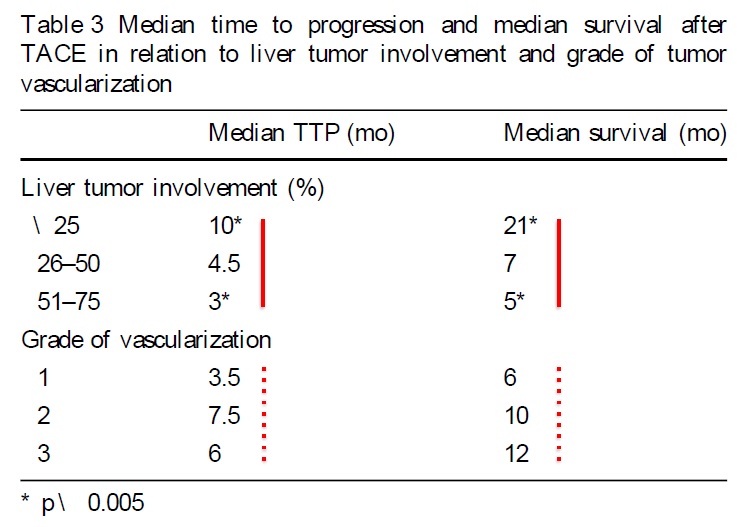
TTP and Survival
Side effects
• 30天死亡率 0%(mortality 0%)
• 腹痛 (Abdominal pain),严重(severe) 32%, 中度(moderate) 40%, 轻度(mild) 28%
• 恶心/呕吐(Nausea/vomiting,ECOG-grading)gade 3 in 12%, grade 2 in 43%, grade 1 in 45%
• 动脉性高压(Arterial hypertension)in 22/71 TACE (31%) syst. RR increased more than 50 mmHg
Can we predict outcome?
Predictors of good outcome
• < 25% 肝脏受累(liver involvement)
• 多血管实质肿瘤(solid tumors with hypervascularization)
• 转移灶内造影剂吸收强度(intense uptake of CM within metastases)
• TACE术后转移灶完全坏死(complete necrosis of mts. after TACE)
• 较短的治疗间隔 (short treatment intervals)
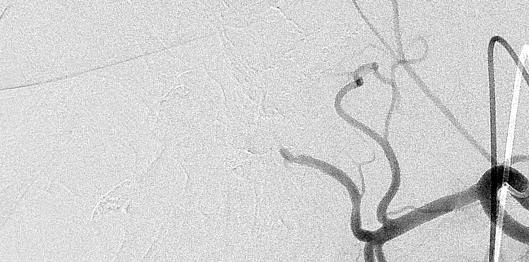
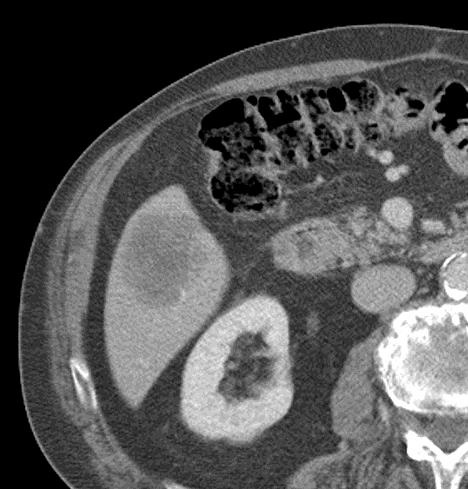
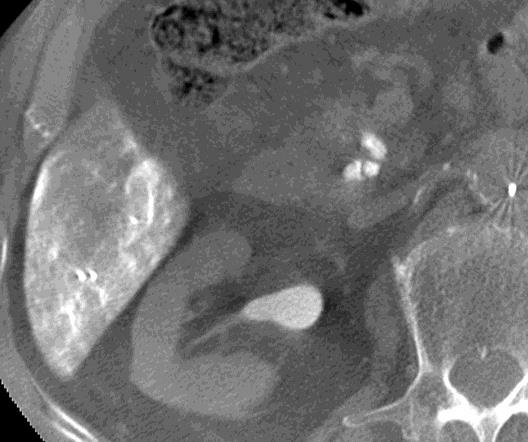
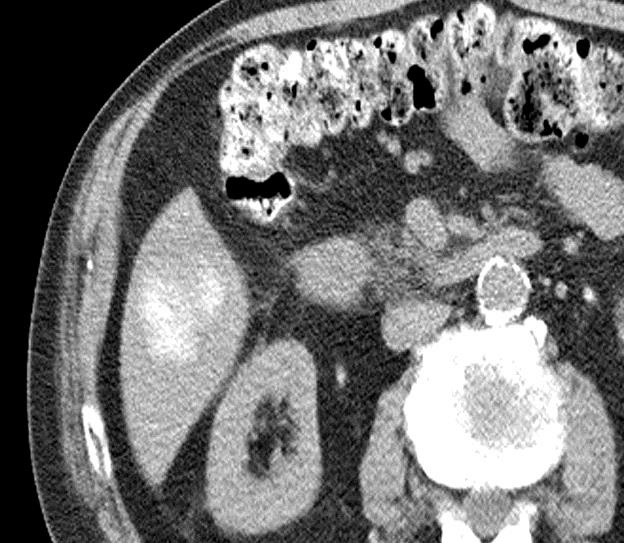
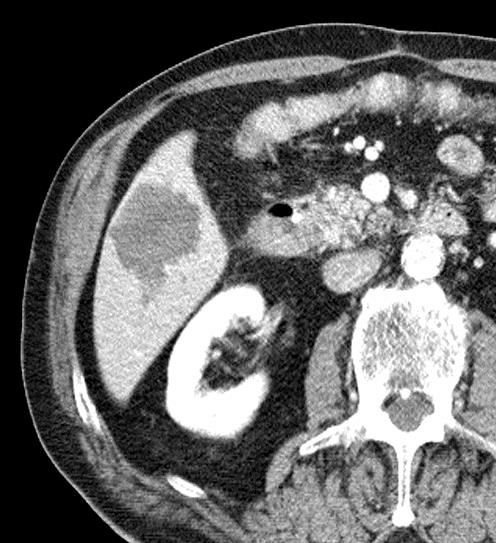
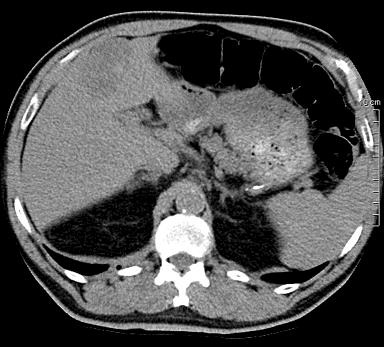
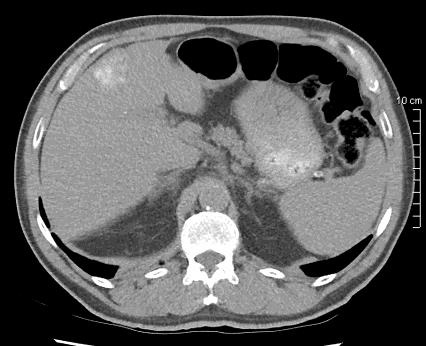
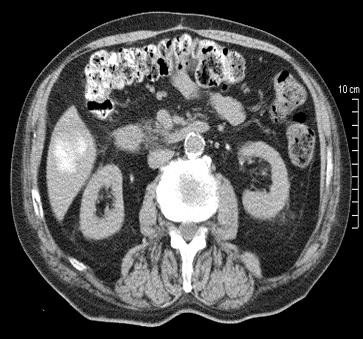
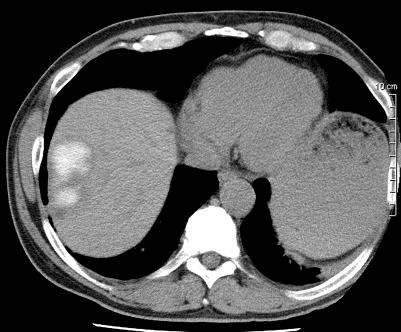
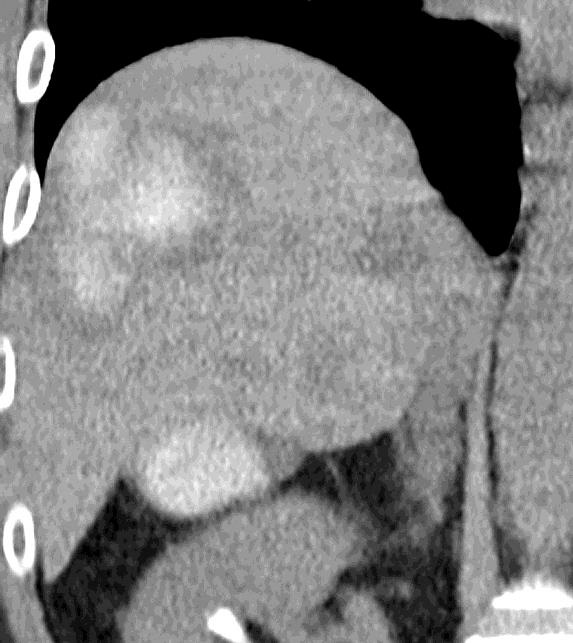
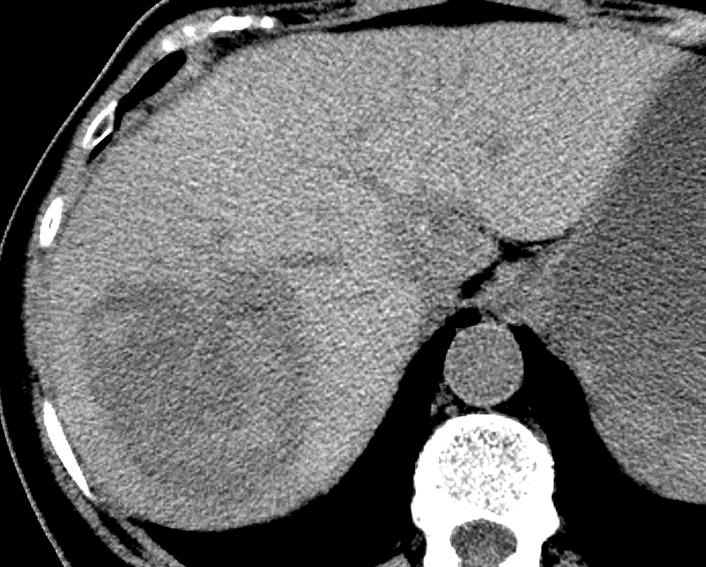
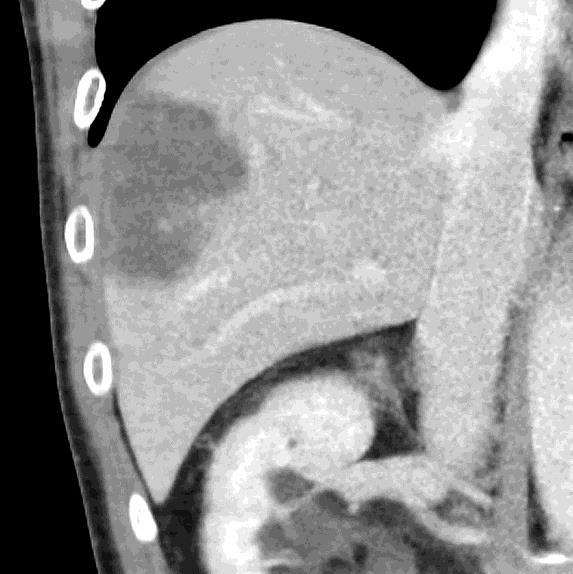
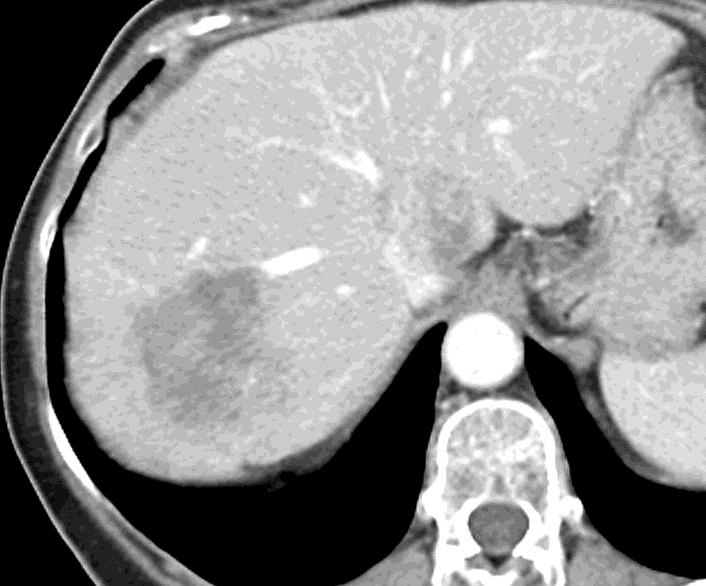
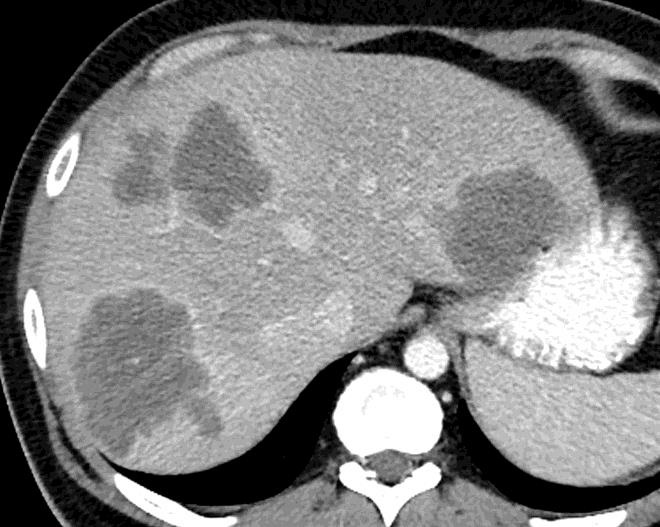
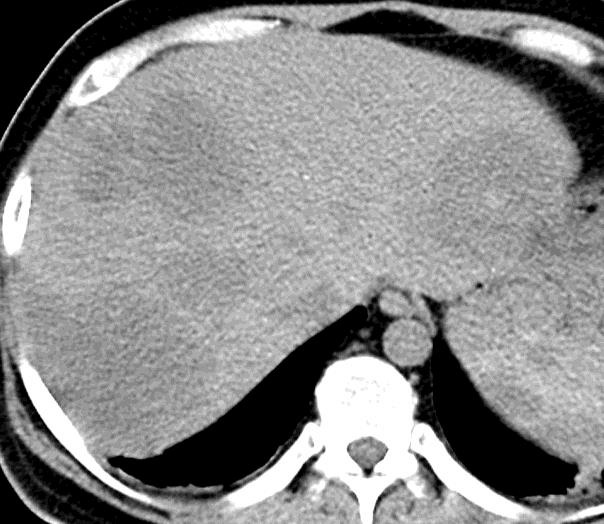
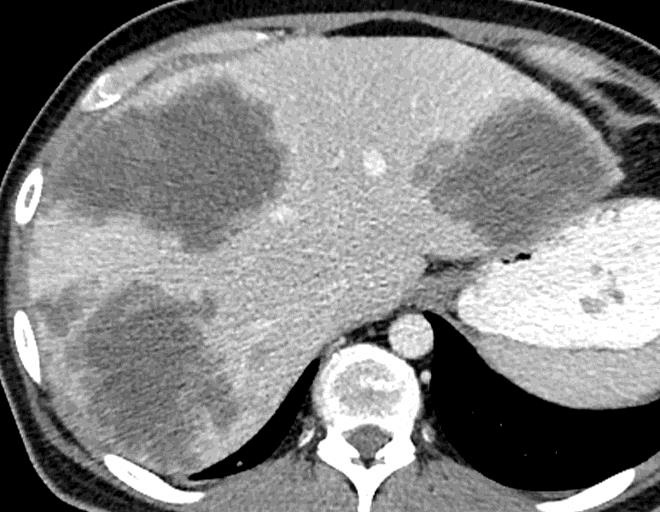
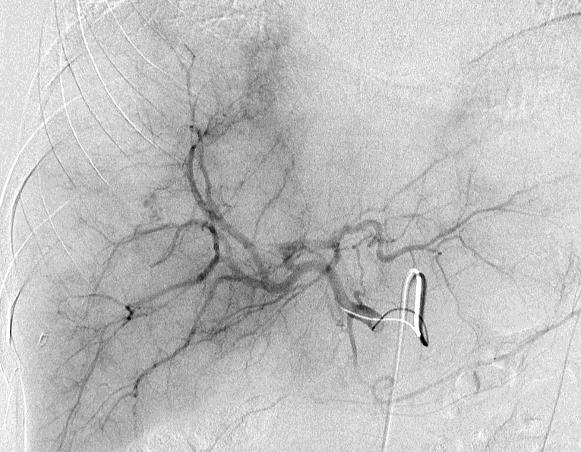
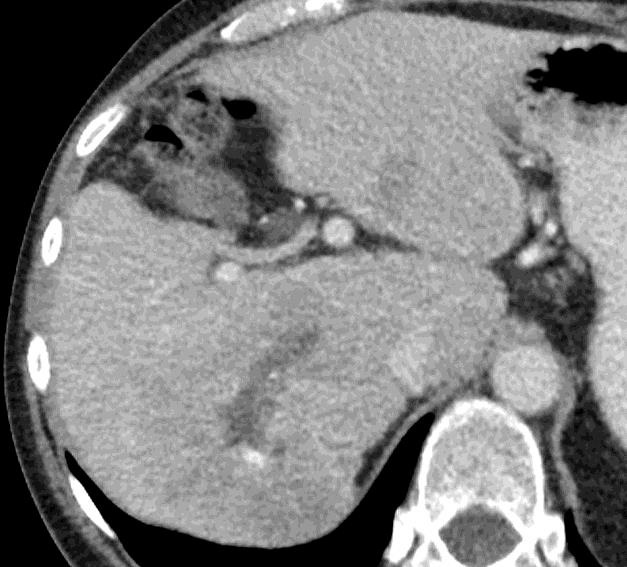
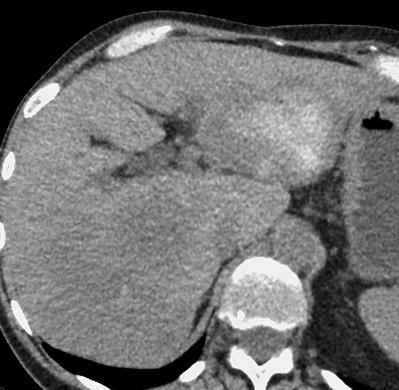
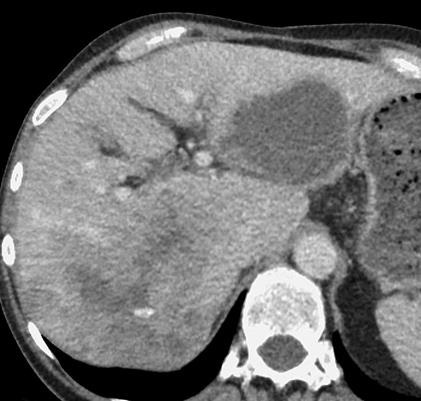
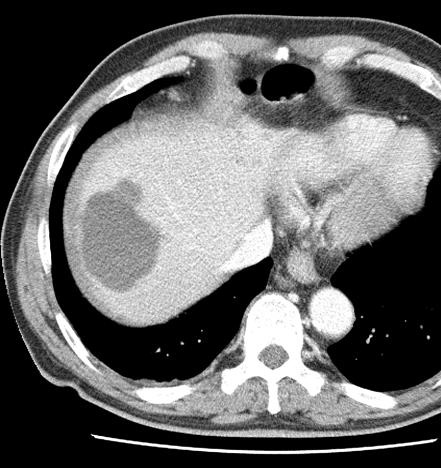
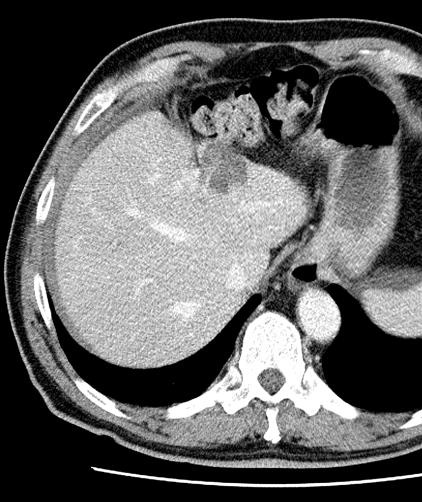
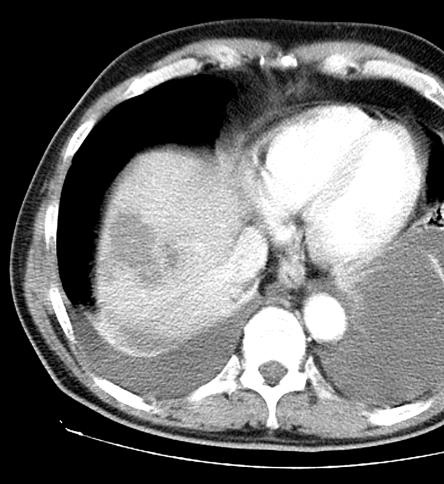
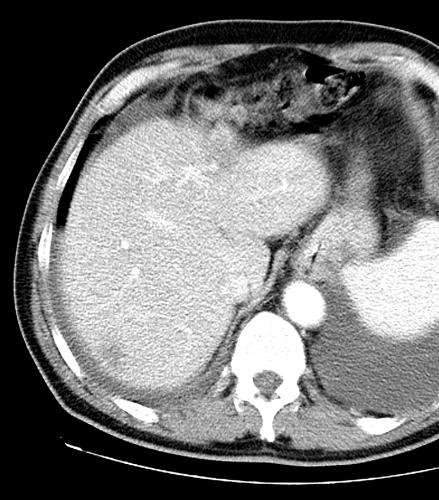
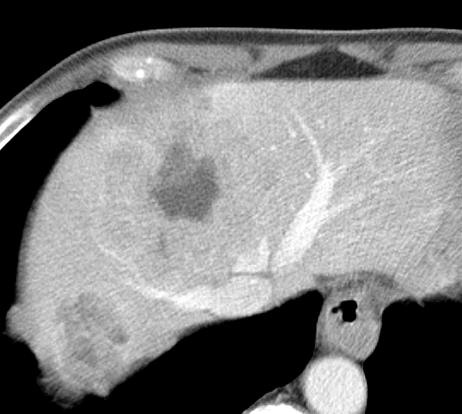
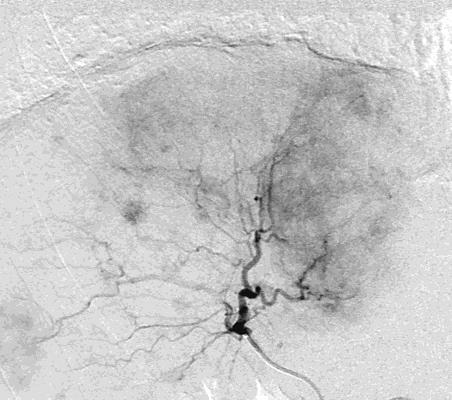
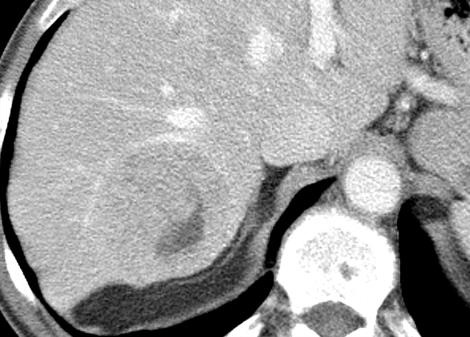
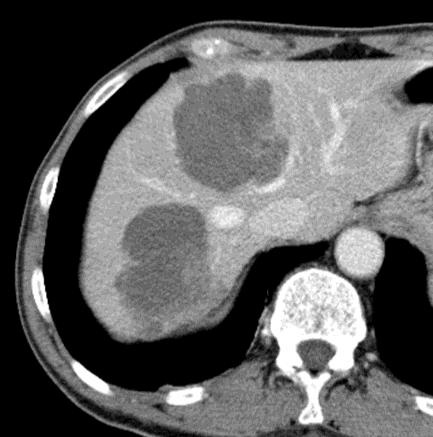
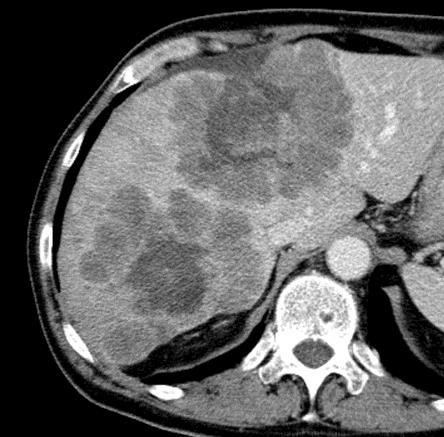
病例介绍1:拯救性化疗
术后3个月
首次术后4年
TACE with Positive Predictors:
TACE with Negative Predictors:
 1. Tellez C, Benson AB 3rd, Lyster MT, Talamonti M, Shaw J, Braun MA, Nemcek AA Jr, Vogelzang RL. Phase II trial of chemoembolization for the treatment of metastatic colorectal carcinoma to the liver and review of the literature. Cancer. 1998 Apr 1;82(7):1250-9. Review. 2. Leichman CG, Jacobson JR, Modiano M, Daniels JR, Zalupski MM, Doroshow JH, Fletcher WS, Macdonald JS. Hepatic chemoembolization combined with systemic infusion of 5-fluorouracil and bolus leucovorin for patients with metastatic colorectal carcinoma: A Southwest Oncology Group pilot trial. Cancer. 1999 Sep 1;86(5):775-81 3. Salman HS, Cynamon J, Jagust M, Bakal C, Rozenblit A, Kaleya R, Negassa A, Wadler S. Randomized phase II trial of embolization therapy versus chemoembolization therapy in previously treated patients with colorectal carcinoma metastatic to the liver.
Clin Colorectal Cancer. 2002 Nov;2(3):173-9.
4. Pwint TP, Midgley R, Kerr DJ. Regional hepatic chemotherapies in the treatment of colorectal cancer metastases to the liver. Semin Oncol. 2010 Apr;37(2):149-59. doi: 10.1053/j.seminoncol.2010.03.005. 5. Huppert P, Wenzel T, Wietholtz H. Transcatheter arterial chemoembolization (TACE) of colorectal cancer liver metastases by irinotecan-eluting microspheres in a salvage patient population. Cardiovasc Intervent Radiol. 2014 Feb;37(1):154-64. |