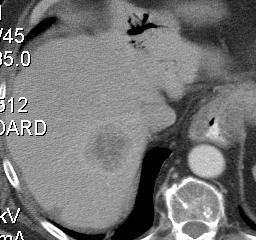
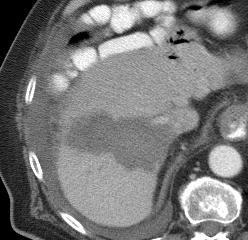
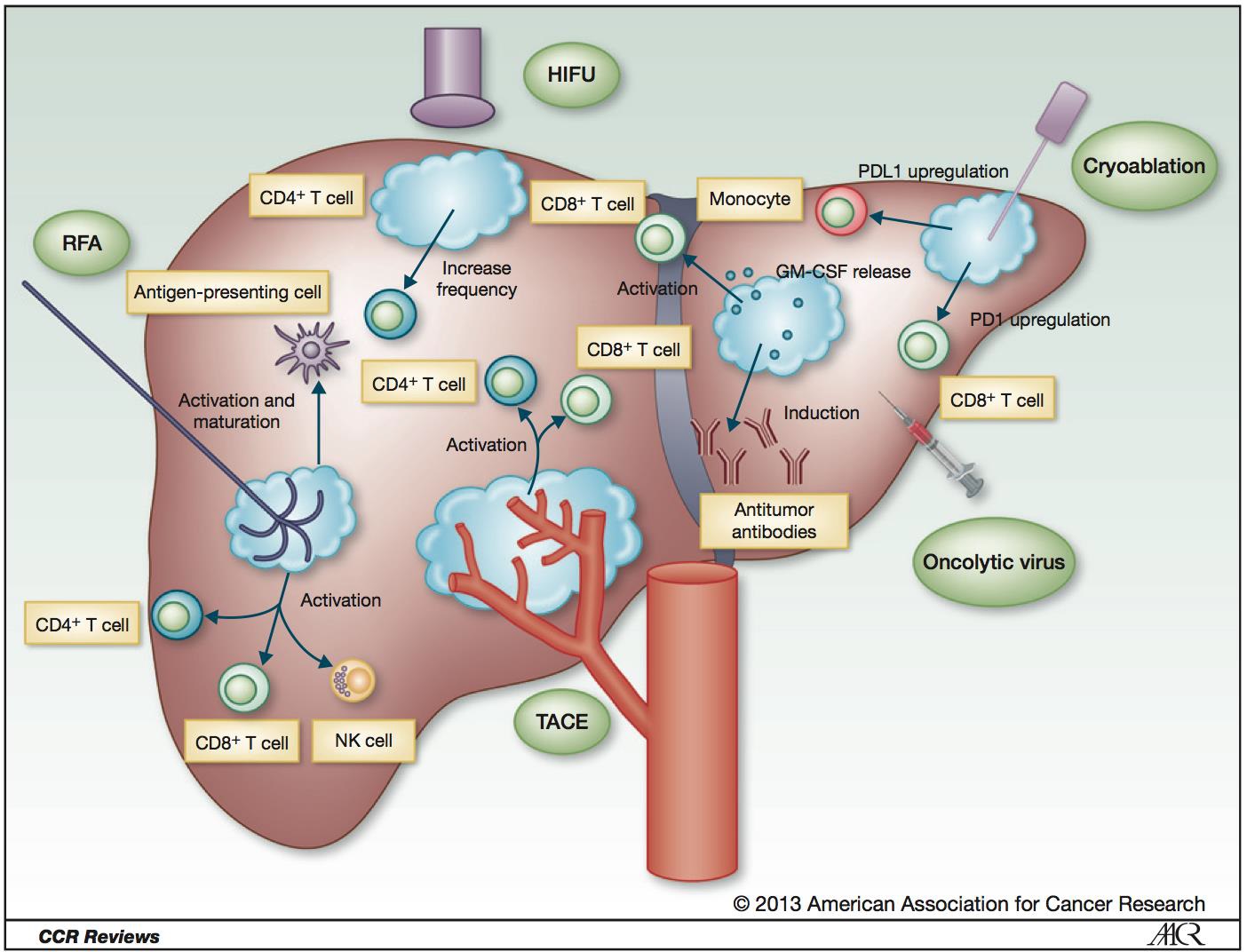
射频消融导致肿瘤特异性免疫反应?这可能潜在成为射频和免疫治疗的一个新的领域。介入治疗对组织和肿瘤的破坏产生的碎片影响人体的免疫系统。肿瘤原位(In-situ)破坏导致抗肿瘤免疫的抗原产生,从而启动肿瘤的免疫治疗。 NIH case (Brad Wood 2009)
原发肿瘤治疗后转移病灶缩小【1】,抗原呈递细胞(Antigen presenting cells)是关键【2】。
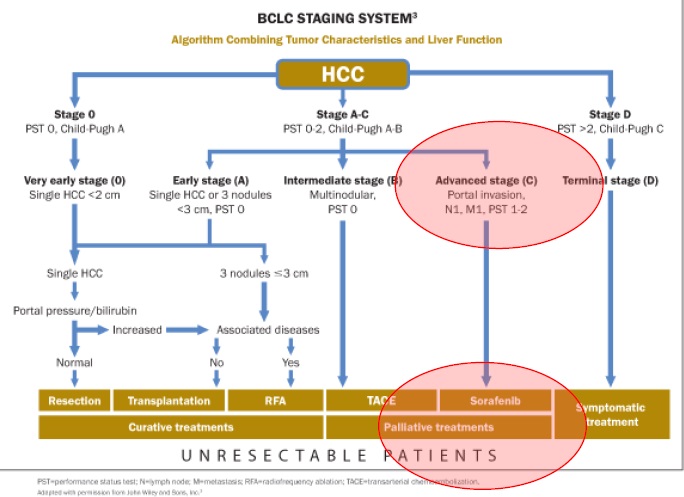
肝癌是炎症(乙肝/丙肝)和潜在非酒精性脂肪肝炎(Nonalcoholic steatohepatitis,NASH)导致的,也是肝移植可治疗的癌症。
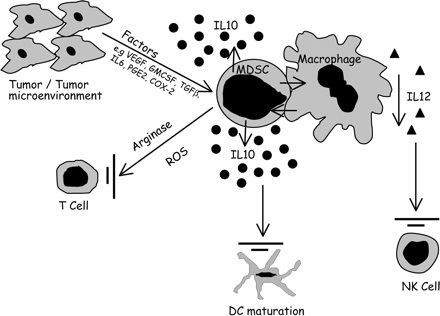
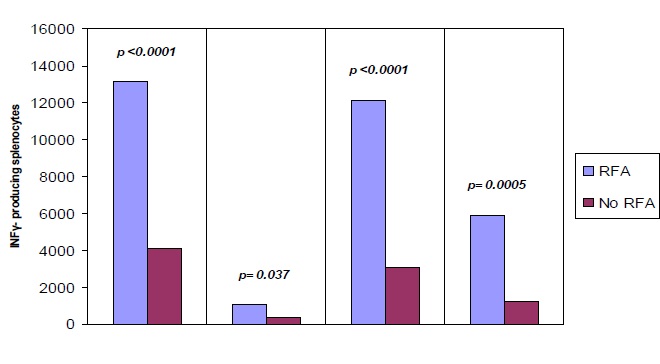
单独射频消融的免疫反应
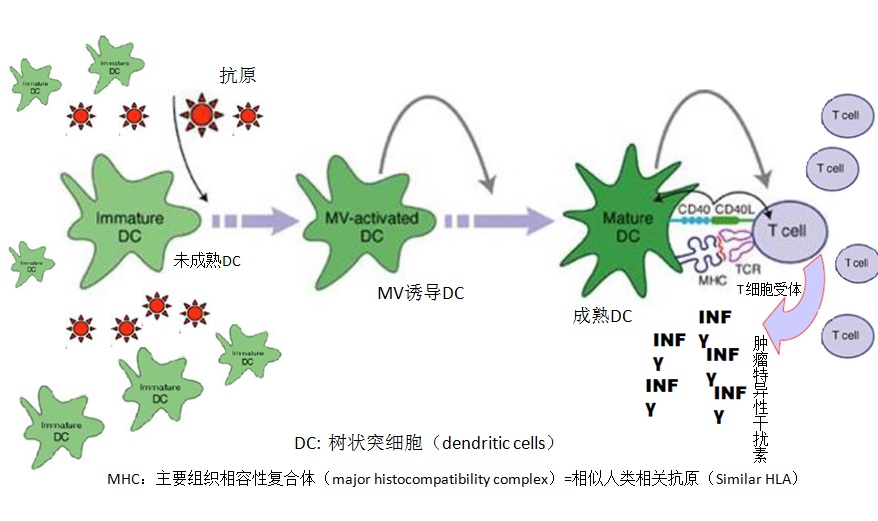
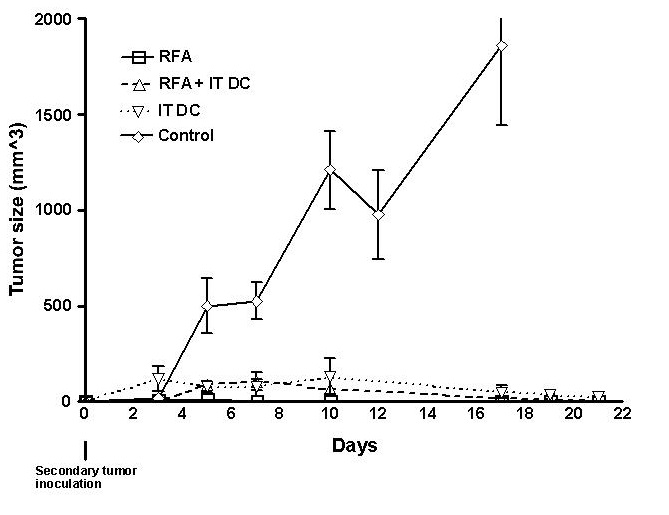
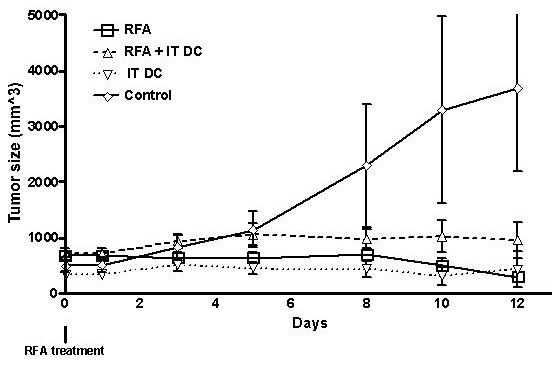
射频消融免疫反应和树突细胞
免疫治疗
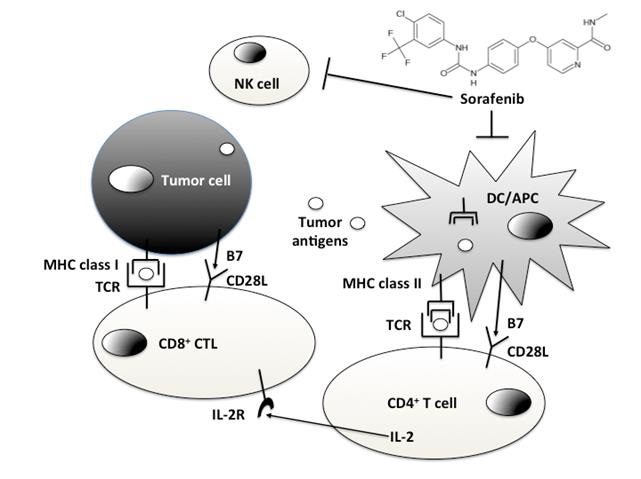
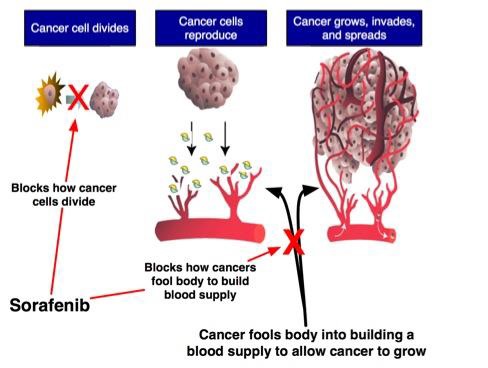
索拉菲尼作为免疫治疗【5】?
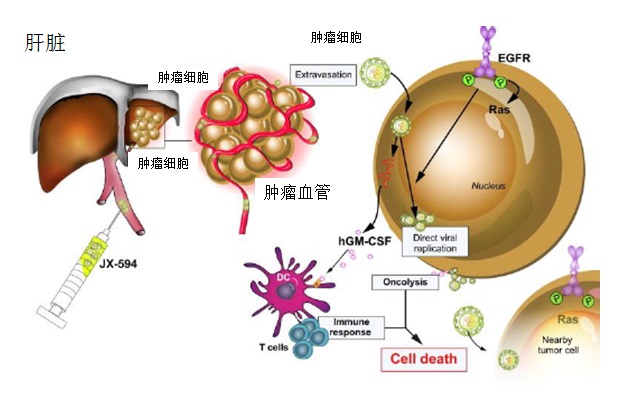
溶瘤细胞病【6,7】
Glypican 3: 磷脂酰肌醇蛋白-3可以用于诊断【8】,也可以用于治疗【9】。 GPC-3是肝癌细胞表面的糖蛋白,在正常人群和肝炎患者的肝细胞均不表达,而在肝癌细胞中高表达。在分化较差的癌组织中过度表达,与预后较差直接相关。 对分化较好的肝癌或纤维板层样肝癌并不适合 GPC3 扮演疾病预后的角色(复发、残余或转移)II 期研究【10】 Anti-Glypican 3: 针对GPC3人源化单克隆抗体,临床前期和临床证实【11-13】
1. Martijn de Brok, et al. In Situ Tumor Ablation Creates an Antigen Source for the Generation of Antitumor Immunity. Cancer Research 64, 4024–4029, 2004. 2. Dromi, et al. Radiofrequency ablation induces antigen-presenting cell infiltration and amplification of weak tumor-induced immunity. Radiology. 2009 Apr;251(1):58-66. 3. Serafini P,Borrello I,Bronte V. Myeloid suppressor cells in cancer: Recruitment,phenotype,properties,and mechanisms of immune suppression [J]. Cancer Biol,2006,16( 1) : 53-65 4. Arihara F, et al. Increased in CD 14 HLA-DR Myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on
prognosis. Cancer Immunology and Immunotherapy 2013;62:1421-30
5. C. Porta1.doi. Review Immunological Effects of Multikinase: A Clue for Integration with Cellular Therapies. J Cancer 2011; 2:333-338. 6. Hernandez-Gea V, Alsinet C, Llovet JM. Oncolytic immunotherapeutic virus in HCC: can it compete with molecular therapies? J Hepatol. 2013 Oct;59(4):882-4. 7. Bartlett DL, Liu Z, Sathaiah M, Ravindranathan R, Guo Z, He Y, Guo ZS. Oncolytic viruses as therapeutic cancer vaccines. Mol Cancer. 2013 Sep 11;12(1):103. doi: 10.1186/1476-4598-12-103. 8. Nadia Iskandar Zakhary, Mervat Sayed Mohamed, Ola Khorshid, Remon Sobhy Azer, Naglaa Zayed Role of glypican-3 in the early diagnosis of hepatocellular carcinoma among Egyptian patients. Journal of Genetic Engineering and Biotechnology Volume 10, Issue 1, June 2012, Pages 73–79 9. Ishiguro T, Sugimoto M, Kinoshita Y, Miyazaki Y, Nakano K, Tsunoda H, Sugo I, Ohizumi I, Aburatani H, Hamakubo T, Kodama T, Tsuchiya M, Yamada-Okabe H. Anti-glypican 3 antibody as a potential antitumor agent for human liver cancer. Cancer Res. 2008 Dec 1;68(23):9832-8.
10. Filmus J, Capurro M. Glypican-3: a marker and a therapeutic target in hepatocellular carcinoma. FEBS J. 2013 May;280(10):2471-6
11. Greten TF, Duffy AG, Korangy F. Hepatocellular carcinoma from an immunologic perspective. Clin Cancer Res. 2013 Dec 15;19(24):6678-85. 12. Sun CK, Chua MS, He J, So SK. Suppression of glypican 3 inhibits growth of hepatocellular carcinoma cells through up-regulation of TGF-β2. Neoplasia. 2011 Aug;13(8):735-47.
13. Feng M, Gao W, Wang R, Chen W, Man YG, Figg WD, Wang XW, Dimitrov DS, Ho M. Therapeutically targeting glypican-3 via a conformation-specific single-domain antibody in hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2013 Mar 19;110(12):E1083-91.
| ||||||||||||||||||||||||