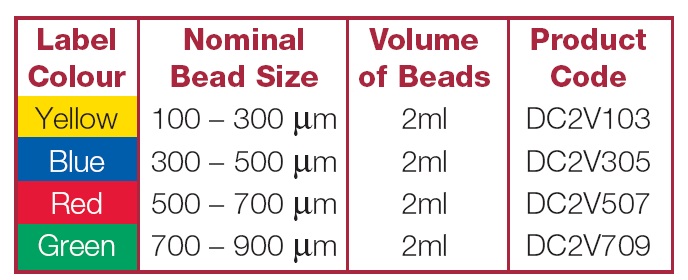
没有标准的TACE方案,文献中TACE使用的药物非常多。比较不同技术的临床试验没有表现出哪一种更具优势。主要应用的药物包括铂类药物、阿霉素类药物和丝裂霉素C等。栓塞剂的使用主要包括常规TACE和药物洗脱微球的栓塞以及放射性微球栓塞。药物洗脱微球局部化疗和暂时性血管阻塞可以导致肿瘤局部区域:1. 较高的药物浓度和药物滞留,2. 少血管区域的血流再分配,3. 增加肝脏的摄取。这三点可以增加治疗的有效性和减少毒性,最终改善生活质量(QOL),增加存活率和由于降低副作用而获得病人最好的顺从性。 产品目前市场上的微球产品:
药物洗脱微球是肝动脉化疗性栓塞的新平台。
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
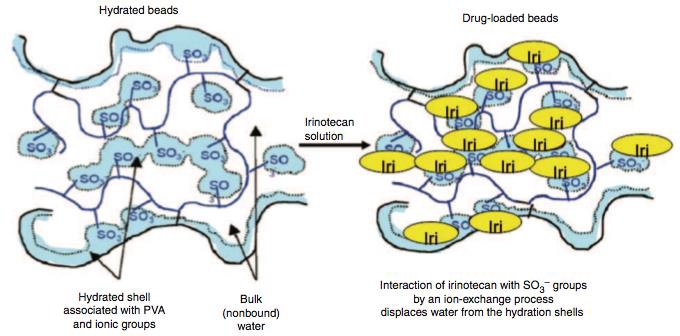 |
| 水化(合)微球与药物荷载微球:将水化微球浸泡在伊立替康溶液中。发生离子交换的过程[1] |
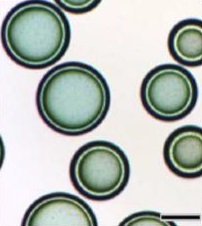
伊立替康(300-500μm)荷载药物(50mg/ML) 镜下观[3]
 |
 |
 |
| 未荷载微球 | 7分钟 | 20分钟 |
DC-Bead是荷载和释放伊立替康药物的一个稳定系统,其稳定性超过28天[1]。微球直径经过精确校准,包括70-150μm、100-300μm和300-500μm大小的微球。通过离子交换荷载伊立替康50mg / 1cc 微球,100mg / 安瓶。在体外吸收药物 93% / 3小时,释放100% / 1周,荷载75%药物时间(t75%)为66分钟[2]。
实验动物模型表明,与静脉或动脉注射伊立替康比较,伊立替康载药微球动脉栓塞血清中伊立替康浓度较低,肿瘤内药物浓度较高和滞留时间较长,肿瘤最大坏死率在24小时左右[4]。
 |
 |
 |
| 静脉注射 = 25% | 动脉注射 = 60% | 伊立替康微球栓塞 = 95% |
I 期临床研究
II 期临床研究
 |
 |
 |
 |
| 作者 | 病人数 | 治疗线 | 药物 | 栓塞剂 | 整体反应率 | PFS(月) | 中位生存期(月) |
| Alibertini(2006)[6] | 10 | TL | 伊立替康 | DC Bead | 75% | N.R. | 12 |
| Florentini(2007)[7] | 20 | TL | 伊立替康 | DC Bead | 65% | 6 | 14 |
| Alibertini(2011)[8] | 82 | STL | 伊立替康 | DC Bead | 78% | 8 | 25 |
| Martin(2012)[9] | 55 | STL | 伊立替康 | DC Bead | 75% | 11 | 19 |
Martin 等人报告55例一或二线化疗失败的结直肠癌肝转移患者进行共99伊立替康药物洗脱微球栓塞(30%的病人同时进行相似的化疗)。副反应发生率28%;无术后30天死亡。反应率在6个月为66%,12个月为75%,中位整体生存率为19个月[9]。
III 期临床研究
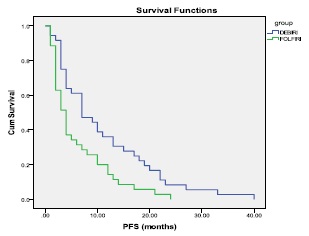
Florentini 等人对74例结直肠癌肝转移化疗抵抗的患者随机分为DEBIRI组(N=36,两次治疗)和FOLFIRI组(N=38,8次化疗)[10]。结果如下:
| DEBIRI(N=35) | FOLFIRI(N=35) | |
| CR+PR | 24(68.6%) | 7(20%) |
| SD | 4(11.4%) | 12(34.3%) |
| PD | 7(20%) | 16(45.7%) |
| 臂 | 平均中位生存期 | PFS | 急性毒性反应 | 后期毒性 | Edmonton 评分 |
欧元/人
|
| DEBIRI(N=34) | 23 | 7 | 70% | 20% | 60% | 5000(2次) |
| FOLFIRI(N=35) | 15 | 4 | 25% | 80% | 22% | 18000(8次) |
Treatment Cost
• 6 months of biweekly 5-FU cost 975 €
• 6 months with FOLFOX/bevacizumab 100.000 €
• 6 months of FOLFIRI cost 35.000 €
• Drug-eluting beads preloaded with IRINOTECAN cost 10.000 €
 |
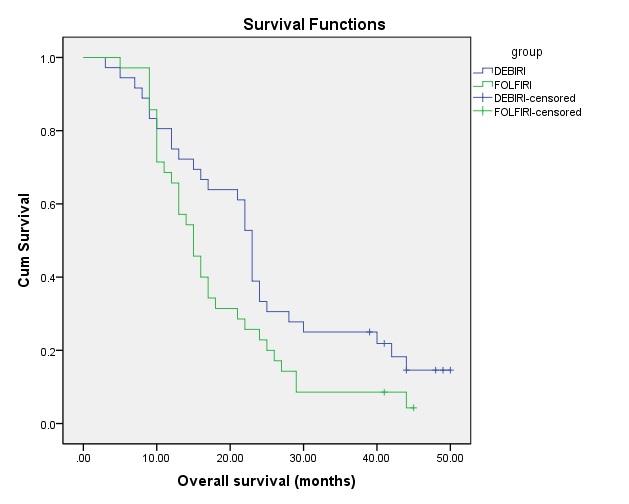 |
| 无进展生存期 | 整体生存率 |
| Giammaria Fiorentini et all ANNALS OF GASTROENTEROLOGY & HEPATOLOGY 2012; 3:(1). March 2012 | |
Mratin 等人报告10 例初始治疗的病人DEBIRI+FOLFOX全身化疗的结果。治疗后9-12个月反应率为100%(2CR,8PR)平均生存期为15.2月,40%的病人降期后得以肝切除或/和射频消融。结论:同时进行DEIRI栓塞和FOLFOX全身化疗用或不用贝伐单抗的结直肠癌肝转移患者安全和有效。副反应轻微,没有毒性限制剂量(dose-limiting toxicities),治疗反应率增加[11]。
可切除肝癌的新辅助治疗[12]
为什么(背景分析)?
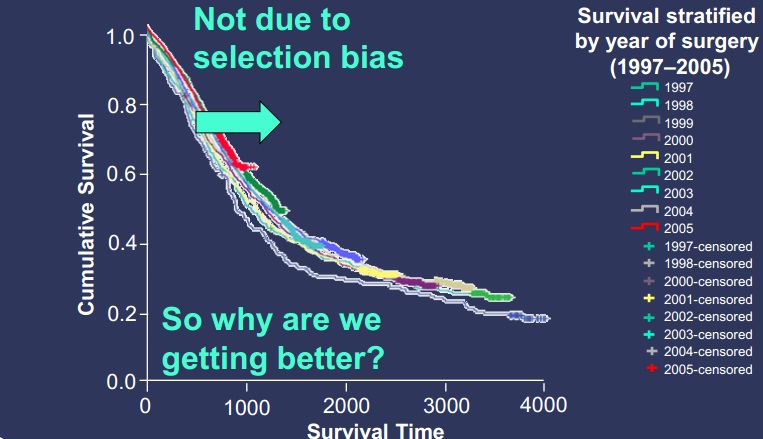 |
| 这么多年没什么进步,所以我们是否可以做到更好? |
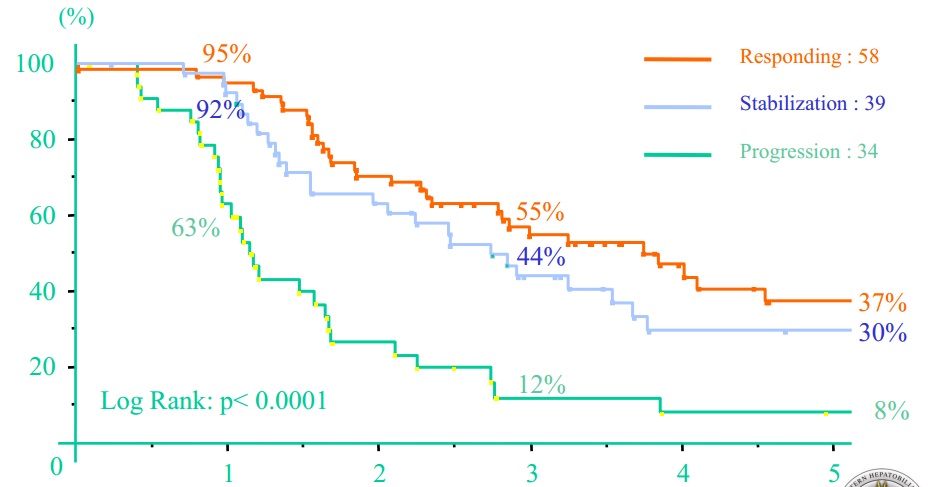 |
| 新辅助化疗似乎效果好一些,但不尽人意 |
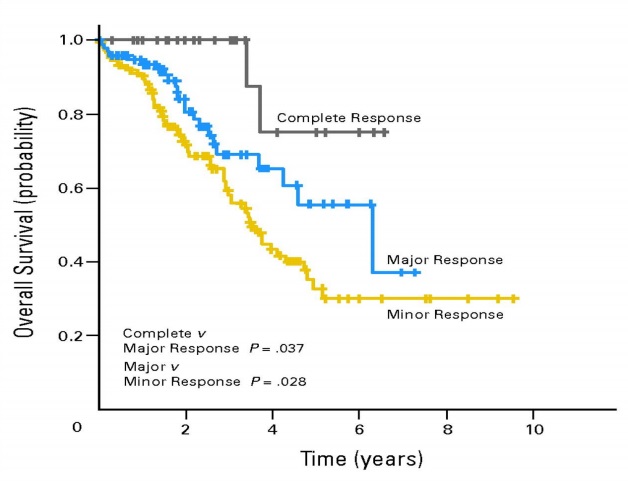 |
||
 |
 |
 |
| 新辅助化疗有效,反应越好生存期越长。肝肿瘤切除标本 | ||
既然可切除肝转移癌术前化疗,所谓新辅助化疗有效,在肝切除后继续化疗成为顺理成章的想法。
Nordlinger 等人[16],对364例可切除肝转移癌进行随机分组,单纯外科切除组和外科切除前和术后化疗组。按RECIST标准CT评价术前化疗后结果显示
CR 7 (3.8%)
PR 73(40.1%)
SD 64(35.2%),8例在3-4次化疗后出现进展,其中3例切除;4例在6次化疗后出现进展,其中1例切除。
没有评价 26(14.3%)
肝切除后无进展生存期
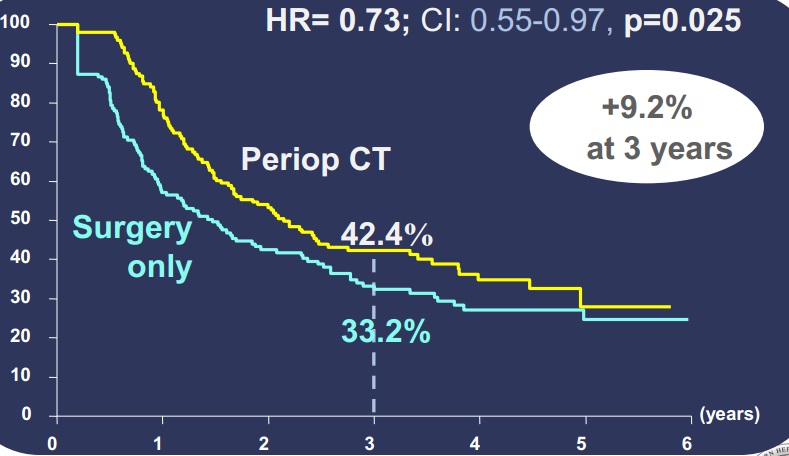 |
其它转移瘤的研究
| 作者/年 | 病例数/原发灶 | 治疗线 | 药物 | 栓塞剂 | 整体反应率% | PFS(月) | 中位生存率(月) |
| Florentini/2009[5] |
N=10 视网膜黑色素瘤 |
TL | 伊立替康 | DC Bead | 90 | 6.5 | 10 |
| Florentini/2012 |
N=52 视网膜黑色素瘤 |
STL | 伊立替康 | DC Bead | 83 | 7.5 | 13.9 |
| Fouad/2013 |
N=13 支气管肺癌 |
一线 |
阿霉素 伊立替康 |
DC Bead | 50 | 14 |
1. Kaiser J, Thiesen J, Krämer I. Stability of irinotecan-loaded drug eluting beads (DC Bead) used for transarterial chemoembolization. J Oncol Pharm Pract. 2010 Mar;16(1):53-61.
2. Jordan O, Denys A, De Baere T, Boulens N, Doelker E. Comparative study of chemoembolization loadable beads: in vitro drug release and physical properties of DC bead and hepasphere loaded with doxorubicin and irinotecan. J Vasc Interv Radiol. 2010 Jul;21(7):1084-90.
3. Taylor RR, Tang Y, Gonzalez MV, Stratford PW, Lewis AL. Irinotecan drug eluting beads for use in chemoembolization: in vitro and in vivo evaluation of drug release properties. Eur J Pharm Sci. 2007 Jan;30(1):7-14. Epub 2006 Sep 15.
4. Olivier Planché, Christophe Teriitehau, Sana Boudabous, Joey Marie Robinson, Pramod Rao, Frederic Deschamps, Geoffroy Farouil, Thierry de Baere. In Vivo Evaluation of Lung Microwave Ablation in a Porcine Tumor Mimic Model. CardioVascular and Interventional Radiology February 2013, Volume 36, Issue 1, pp 221-228
5. Fiorentini G, Aliberti C, Del Conte A, Tilli M, Rossi S, Ballardini P, Turrisi G, Benea G. Intra-arterial hepatic chemoembolization (TACE) of liver metastases from ocular melanoma with slow-release irinotecan-eluting beads. Early results of a phase II clinical study. In Vivo. 2009 Jan-Feb;23(1):131-7.
6. Aliberti C, Tilli M, Benea G, Fiorentini G. Trans-arterial chemoembolization (TACE) of liver metastases from colorectal cancer using irinotecan-eluting beads: preliminary results. Anticancer Res. 2006 Sep-Oct;26(5B):3793-5.
7. Fiorentini G, Aliberti C, Turrisi G, Del Conte A, Rossi S, Benea G, Giovanis P. Intraarterial hepatic chemoembolization of liver metastases from colorectal cancer adopting irinotecan-eluting beads: results of a phase II clinical study. In Vivo. 2007 Nov-Dec;21(6):1085-91.
9. Martin RC, Joshi J, Robbins K, Tomalty D, Bosnjakovik P, Derner M, Padr R, Rocek M, Scupchenko A, Tatum C. Hepatic intra-arterial injection of drug-eluting bead, irinotecan (DEBIRI) in unresectable colorectal liver metastases refractory to systemic chemotherapy: results of multi-institutional study. Ann Surg Oncol. 2011 Jan;18(1):192-8. doi: 10.1245/s10434-010-1288-5. Epub 2010 Aug 26.(原文)
10. Giammaria Fiorentini, Camillo Aliberti, Giorgio Benea , Massimo Tilli, Francesco Graziano, Paolo Coschiera, Andrea Mambrini, Maurizio Cantore, and Stefano Guadagni. Hepatic Arterial Chemoembolization Adopting Dc Bead† , Drug-Eluting Bead Loaded with Irinotecan (Debiri) Versus Systemic Therapy for Hepatic Metastases from Colorectal Cancer: A Randomized Study of Efficacy and Quality of Life. ANNALS OF GASTROENTEROLOGY & HEPATOLOGY 2012; 3:(1) 39-48. March 2012
11. Martin RC 2nd, Scoggins CR, Tomalty D, Schreeder M, Metzger T, Tatum C, Sharma V. Irinotecan drug-eluting beads in the treatment of chemo-naive unresectable colorectal liver metastasis with concomitant systemic fluorouracil and oxaliplatin: results of pharmacokinetics and phase I trial. J Gastrointest Surg. 2012 Aug;16(8):1531-8
12. http://assets.biocompatibles.com/products/uploads/Literature/2010/12/Poston.pdf
11. Martin RC 2nd, Scoggins CR, Tomalty D, Schreeder M, Metzger T, Tatum C, Sharma V. Irinotecan drug-eluting beads in the treatment of chemo-naive unresectable colorectal liver metastasis with concomitant systemic fluorouracil and oxaliplatin: results of pharmacokinetics and phase I trial. J Gastrointest Surg. 2012 Aug;16(8):1531-8
12. http://assets.biocompatibles.com/products/uploads/Literature/2010/12/Poston.pdf
13. Morris EJ, Forman D, Thomas JD, Quirke P, Taylor EF, Fairley L, Cottier B, Poston G. Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg. 2010 Jul;97(7):1110-8.
14. Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, Giacchetti S, Paule B, Kunstlinger F, Ghémard O, Levi F, Bismuth H. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004 Oct;240(4):644-57; discussion 657-8.
15. Blazer DG 3rd, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, Fogelman D, Eng C, Chang DZ, Wang H, Zorzi D, Ribero D, Ellis LM, Glover KY, Wolff RA, Curley SA, Abdalla EK, Vauthey JN. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008 Nov 20;26(33):5344-51. doi: 10.1200/JCO.2008.17.5299. Epub 2008 Oct 20.
15. Blazer DG 3rd, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, Fogelman D, Eng C, Chang DZ, Wang H, Zorzi D, Ribero D, Ellis LM, Glover KY, Wolff RA, Curley SA, Abdalla EK, Vauthey JN. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008 Nov 20;26(33):5344-51. doi: 10.1200/JCO.2008.17.5299. Epub 2008 Oct 20.
16. Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Collette L, Praet M, Bethe U, Van Cutsem E, Scheithauer W, Gruenberger T; EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD). Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008 Mar 22;371(9617):1007-16.