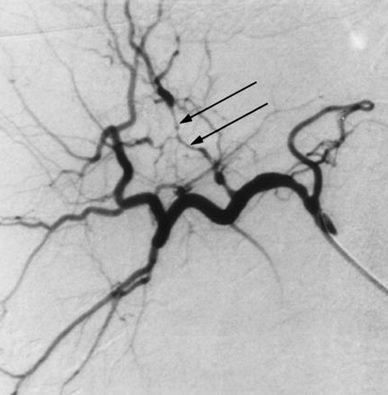
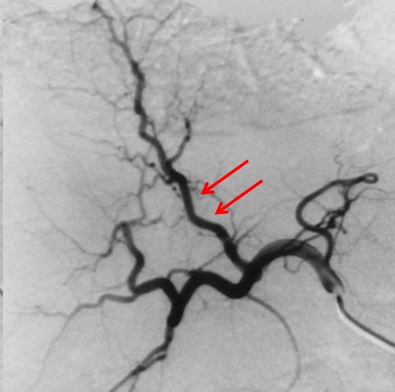
| 一、技术相关并发症 1. 动脉痉挛
二、栓塞并发症 TACE严重并发症
所谓严重并发症的定义是指 需要特殊治疗或/和导致延长住院时间 (1)死亡:一共34篇文献中报告过TACE相关死亡的病例(术后30天内)。其中7/34 篇文献报告治疗相关死亡率为0%,27/34 报告治疗相关死亡率为0.5%~17%。 TACE相关死亡的原因 - 肝衰竭(报告死亡原因>60%) - 肿瘤破裂 - 感染性休克 - 消化道出血 - 肾功能衰竭 - 肝性脑病 - 肝脓肿 - 十二指肠溃疡穿孔 - 缺血性结肠穿孔 - 门静脉血栓 - 呼吸衰竭 其中肝衰竭、胃肠道出血、肾功能衰竭和肝性脑病是由于肝硬化失代偿所致;而肿瘤破裂,肝脓肿、十二指肠溃疡穿孔,结肠穿孔、门静脉血栓和呼吸衰竭可能和TACE本身有关。 (2)TACE的其它并发症 1)全身并发症:主要指栓塞后综合症。包括腹痛,发烧和恶心。占所治疗病人的35%[8]~100%[9,10]。通常是暂时的,大约2周左右。发烧的时间肿瘤的大小、基线AST、以及治疗的次数有关[11]。而其严重性(需要止痛药>7天)主要和肿瘤的大小有关[12,13]。 2)肝功能失代偿:肝功能损害和食道静脉曲张出血以及肾功能衰竭。很多回顾性研究和临床试验都比较难定界定这一概念。任何从暂时性转氨酶升高到导致死亡的肝功能急性衰竭。发生率22%-67%[14]。这一数值显得较高。需要更精确的定义,比如黄疸、腹水、肝性脑病或静脉曲张出血。肝动能失代偿的潜在机制包括肝动脉栓塞后高度依赖动脉的硬化肝有效血流被减少、炎症反应至急性肿瘤坏死以及减少了肝功能的储备。黄疸(总胆红素升高≥2mg/dL)发生率为15%[11];食道静脉曲张出血发生率1-1.5%[11,15,16]。腹水1%[11,15],肝性脑病[11]。
No significant changes in HPVG 3 days post-TACE[15].
– Mean: 13.1 mmHg pre vs. 12.8 mmHg post
肝功能异常失代偿主要和肝功能基线数据差有关,如 Child 分级,ICG R15 >20等[11,17-20]。另外与肿瘤较大有关[19]。在栓塞前有腹水的病人当中[18],17%有早期肝衰竭(94% 生存期不到12个月)。肝衰竭独立的预测因素是Child-Pugh 评分(OR: 10.1)和栓塞后消化道出血(OR 10.8)。 3)肾功能失代偿(定义,肌酐增加>25?):大样本(100-500例)研究显示 TACE术后发生肾功能失代偿的比率为3,3%-9%[21,22] 。仅有68%是暂时的,15%的病例以前有肾功能不全(eGFR<60ml/min/1.73m²)。独立的预测因子为术后消化道出血(OR:16.5)和CLIP评分≥2(OR:2.1)[21]。发生急性肾功能衰竭的病例与未发生急性肾功能衰竭的病例比较住院相关死亡率30% vs 1.5%(P<0.001,OR: 26.1)。 4)局部并发症:肿瘤破裂,脓肿形成;胆道损伤,动脉夹层,动脉阻塞和假性动脉瘤 肿瘤破裂发生几率低(0.1-1%)但它是致命并发症。TACE术后肿瘤破裂发生时间充满变数,但多数病人是首次进行栓塞治疗。破裂导致的急性出血和慢性肝功能衰竭是直接死亡原因[14,23,24]。 栓塞后脓肿形成的整体发生率据报告≤2.6%。不同肝肿瘤发生率又不同,其中肝细胞肝癌占0.0-0.7%,肝转移癌占7-12%,胆管细胞癌占10-25%。但死亡率高达50%。培养显示多为肠源性细菌。高危人群是胆道壶腹部支撑架植入或胆管改道手术的病人(RR≈894)。使用预防性的抗菌素充满争议,一项RCT研究不能证实肝细胞肝癌栓塞后获益。对于高危病人更为积极的方案他唑巴坦(tazobactam)/ 哌拉西林(piperacillin)+新霉素(neomycin)/红霉素(erythromycin)优于标准的头孢苄胺静脉注射[14,16,24]。 胆道损伤,包括胆囊炎、胆囊梗死和胆管缺血。胆管炎和胆囊梗死的发生率在0.8%-5.5%之间。主要由于栓塞剂返流或由于即使是导管头在胆囊动脉远端,但存在副胆囊动脉返流。早期外科干预治疗或经皮胆囊引流术可以拯救患者的生命。胆管缺血的发生率在1.1%-6.9%之间。栓塞微粒越小(50-100μm的校准微球)发生胆道缺血的可能性就越大。主要表现为胆汁瘤(biloma),胆管局限性狭窄和弥漫性轻度扩张[14,16]。 5)远处并发症:肝外栓塞和胸膜渗出(胸水) 肝外栓塞主要是由于存在动静脉瘘导致栓塞肝脏时出血肺栓塞,栓塞剂返流导致胰腺栓塞[28],或由于右到左分流而出血脑栓塞[25,26]。 肝动脉栓塞并发症的预防 选择理想的病人 - 肝功能(没有腹水,总胆红素≤2mg/dl,Child-Pugh ≤7) - 肾功能(避免GFR<60ml/min/1.73m²) - 较少伴发疾病,至少可以进行1-2次的选择性治疗 - 被栓塞的肿瘤不宜过大,< 7-10cm - 充分的门静脉血流 - 尽量没有胆汁污染的可能,如过壶腹部的胆道支撑架或胆道改道手术(Whipple 等) 对于治疗失败或复发肿瘤 - 按需治疗 - 注意肝功能下降 - 技术上可行并不意味着对患者有益
1. Cammà C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, Andreone P, Craxì A, Cottone M. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002 Jul;224(1):47-54.
2. Poon RT, Tso WK, Pang RW, Ng KK, Woo R, Tai KS, Fan ST. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol. 2007 Sep;5(9):1100-8. 3. Hsieh CB, Chang HM, Chen TW, Chen CJ, Chan DC, Yu JC, Liu YC, Chang TM, Shen KL.Comparison of transcatheter arterial chemoembolization, laparoscopic radiofrequency ablation, and conservative treatment for decompensated cirrhotic patients with hepatocellular carcinoma. World J Gastroenterol. 2004 Feb 15;10(4):505-8. 4. Kirchhoff TD,et al Transarterial chemoembolization using degradable starch microspheres and iodized oil in the treatment of advanced hepatocellular carcinoma: evaluation of tumor response, toxicity, and survival. Hepatobiliary Pancreat Dis Int. 2007 Jun;6(3):259-66.
5. Pelletier G, Roche A, Ink O, Anciaux ML, Derhy S, Rougier P, Lenoir C, Attali P, Etienne JP. A randomized trial of hepatic arterial chemoembolization in patients with unresectable hepatocellular carcinoma. J Hepatol. 1990 Sep;11(2):181-4.
6. Molinari M, Kachura JR, Dixon E, Rajan DK, Hayeems EB, Asch MR, Benjamin MS, Sherman M, Gallinger S, Burnett B, Feld R, Chen E, Greig PD, Grant DR, Knox JJ. Transarterial chemoembolisation for advanced hepatocellular carcinoma: results from a North American cancer centre. Clin Oncol (R Coll Radiol). 2006 Nov;18(9):684-92. 7. Savastano S, Miotto D, Casarrubea G, Teso S, Chiesura-Corona M, Feltrin GP. Transcatheter arterial chemoembolization for hepatocellular carcinoma in patients with Child's grade A or B cirrhosis: a multivariate analysis of prognostic factors. J Clin Gastroenterol. 1999 Jun;28(4):334-40. 8. Jordi Bruix, Josep M. Llovet, Antoni Castells, Xavier Montañá, Concepció Brú, Maria Del Carmen Ayuso, Ramon Vilana and Joan Rodés. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: Results of a randomized, controlled trial in a single institution. Hepatology Volume 27, Issue 6, June 1998, Pages: 1578–1583, 9. Bayraktar Y, Balkanci F, Kayhan B, Uzunalimoglu B, Gokoz A, Ozisik Y, Gurakar A, Van Thiel DH, Firat D. A comparison of chemoembolization with conventional chemotherapy and symptomatic treatment in cirrhotic patients with hepatocellular carcinoma. Hepatogastroenterology. 1996 May-Jun;43(9):681-7. 10. Hsieh MY, Chang WY, Wang LY, Chen SC, Chuang WL, Lu SN, Wu DK. Treatment of hepatocellular carcinoma by transcatheter arterial chemoembolization and analysis of prognostic factors. Cancer Chemother Pharmacol. 1992;31 Suppl:S82-5. 11. Chan AO, Yuen MF, Hui CK, Tso WK, Lai CL.A prospective study regarding the complications of transcatheter intraarterial lipiodol chemoembolization in patients with hepatocellular carcinoma. Cancer. 2002 Mar 15;94(6):1747-52. 12. Ahrar K, Gupta S. Hepatic artery embolization for hepatocellular carcinoma: technique, patient selection, and outcomes. Surg Oncol Clin N Am. 2003 Jan;12(1):105-26. 13. J W Chung, J H Park, J K Han, B I Choi, M C Han, H S Lee, and C Y Kim Hepatic tumors: predisposing factors for complications of transcatheter oily chemoembolization. Radiology January 1996 198:33-40 14. Zhengang Sun, Gaopeng Li, Xi Ai, Baoming Luo, Yanling Wen, Zizhuo Zhao, Shengli Dong, Jian Guan. Hepatic and biliary damage after transarterial chemoembolization for malignant hepatic tumors: Incidence, diagnosis, treatment, outcome and mechanism. Critical Reviews in Oncology/Hematology, Volume 79, Issue 2, August 2011, Pages 164-174 15. Chiara E, et al. J Gastroenterol Hepatol. 2011;23:573. 16. Malagari K, Pomoni M, Spyridopoulos TN, Moschouris H, Kelekis A, Dourakis S, Alexopoulou E, Koskinas J, Angelopoulos M, Kornezos J, Pomoni A, Tandeles S, Marinis A, Rizos S, Kelekis D. Safety profile of sequential transcatheter chemoembolization with DC Bead™: results of 237 hepatocellular carcinoma (HCC) patients. Cardiovasc Intervent Radiol. 2011 Aug;34(4):774-85. 17. Hwang JI, Chow WK, Hung SW, Li TC, Cheng YP, Ho YJ, Lin CC. Development of a safety index of transarterial chemoembolization for hepatocellular carcinoma to prevent acute liver damage. Anticancer Res. 2005 May-Jun;25(3c):2551-4.
18. Chen MS, Li JQ, Zhang YQ, Lu LX, Zhang WZ, Yuan YF, Guo YP, Lin XJ, Li GH. High-dose iodized oil transcatheter arterial chemoembolization for patients with large hepatocellular carcinoma. World J Gastroenterol. 2002 Feb;8(1):74-8.
19. Poon RT, Ngan H, Lo CM, Liu CL, Fan ST, Wong J. Transarterial chemoembolization for inoperable hepatocellular carcinoma and postresection intrahepatic recurrence. J Surg Oncol. 2000 Feb;73(2):109-14. 20. Shah SR, Riordan SM, Karani J, Williams R Tumour ablation and hepatic decompensation rates in multi-agent chemoembolization of hepatocellular carcinoma. QJM. 1998 Dec;91(12):821-8. 21. Hsin, I-Fang; Hsu, Chia-Yang; Huang, Hui-Chun; Huang, Yi-Hsiang; Lin, Han-Chieh; Lee, Rheun-Chuan; Chiang, Jen-Huey; Lee, Fa-Yauh; Huo, Teh-Ia; Lee, Shou-Dong. Liver Failure After Transarterial Chemoembolization for Patients With Hepatocellular Carcinoma and Ascites: Incidence, Risk Factors, and Prognostic Prediction. Journal of Clinical Gastroenterology. 45(6):556-562, July 2011. 22. Cho HS, Seo JW, Kang Y, Bae EJ, Kim HJ, Chang SH, Park DJ. Incidence and risk factors for radiocontrast-induced nephropathy in patients with hepatocellular carcinoma undergoing transcatheter arterial chemoembolization. Clin Exp Nephrol. 2011 Oct;15(5):714-9. 23. Poggi G, Pozzi E, Riccardi A, Tonini S, Montagna B, Quaretti P, Tagliaferri B, Sottotetti F, Baiardi P, Pagella C, Minoia C, Bernardo G. Complications of image-guided transcatheter hepatic chemoembolization of primary and secondary tumours of the liver. Anticancer Res. 2010 Dec;30(12):5159-64 24. Halpenny DF, Torreggiani WC. The infectious complications of interventional radiology based procedures in gastroenterology and hepatology. J Gastrointestin Liver Dis. 2011 Mar;20(1):71-5. 26 .Lee CS, Kim SJ, Choi JW, Choi CG, Lee DH. Cerebral lipiodol embolism proven by dual-energy computed tomography: a case report. J Comput Assist Tomogr. 2010 Jan;34(1):105-6 27. Bilbao JI, Martínez-Cuesta A, Urtasun F, Cosín O. Complications of embolization. Semin Intervent Radiol. 2006 Jun;23(2):126-42 28. Bae SI, Yeon JE, Lee JM, Kim JH, Lee HJ, Lee SJ, Suh SJ, Yoon EL, Kim HR, Byun KS, Seo TS. A case of necrotizing pancreatitis subsequent to transcatheter arterial chemoembolization in a patient with hepatocellular carcinoma. Clin Mol Hepatol. 2012 Sep;18(3):321-5 |