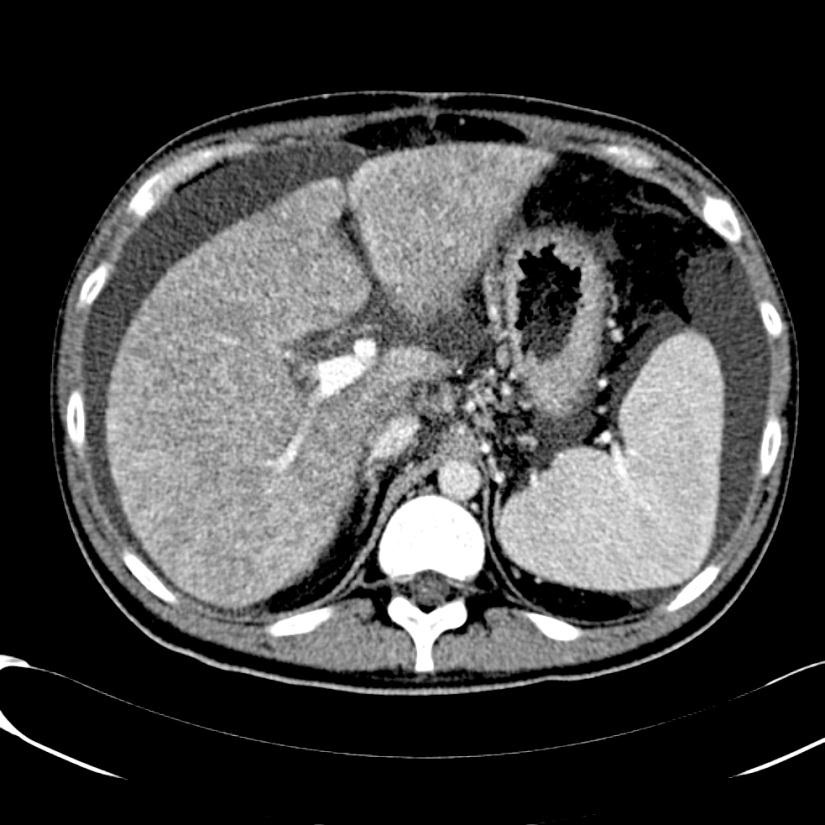
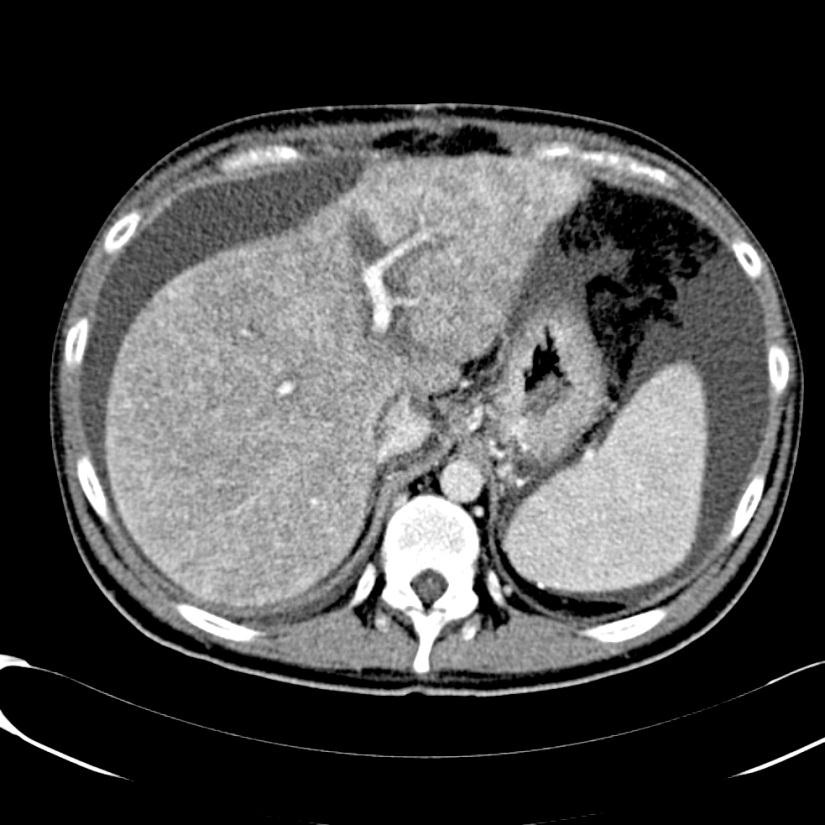
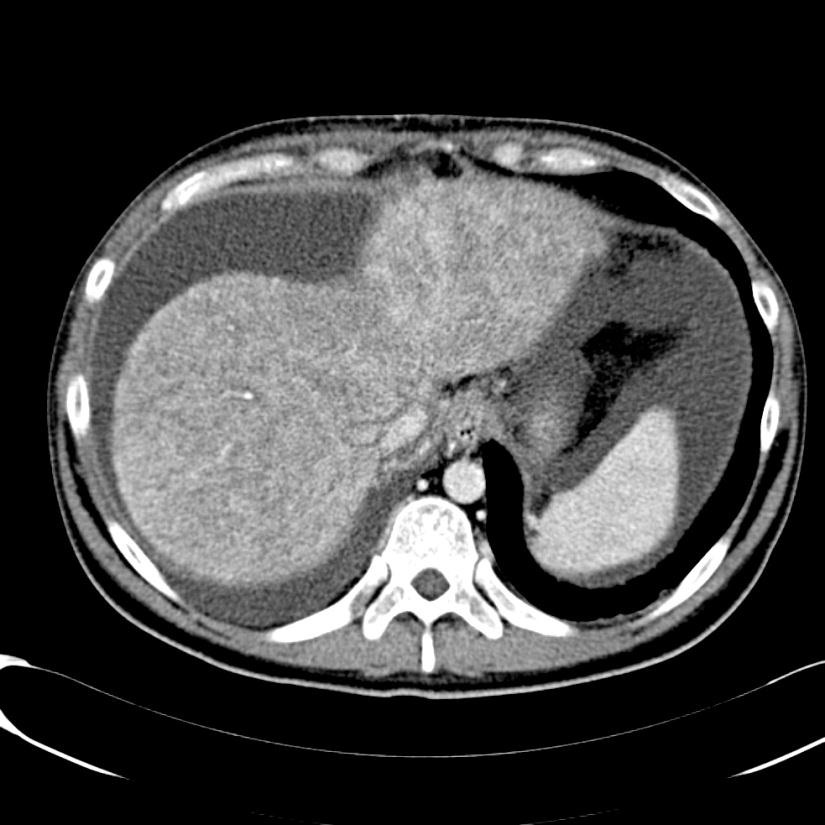
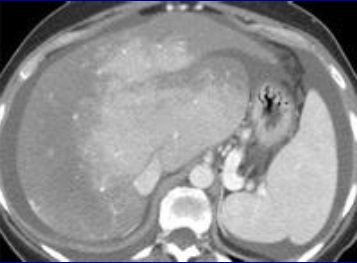
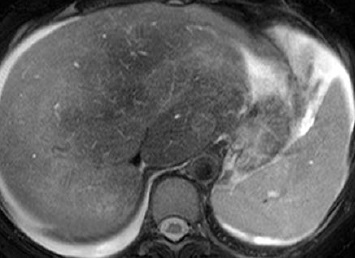
随着现代的成像技术出现,目前大多数情况下BCS患者的诊断通过非侵入性成像。放射科医生的经验和对BCS临床怀疑保持警觉是非常重要的。理想的影像学方法应该显示肝静脉、下腔静脉和门静脉的解剖。超声、CT和MRI使最常用的检查方法。
BCS影像学发现的关键点是:
-
肝静脉或/和下腔静脉阻塞(Occlusion of hepatic veins and/or v. cava)
-
肝尾叶肥大( Caudate lobe enlargement)
-
非均匀的肝实质增强( Inhomogeneous liver enhancement)
-
肝内侧支循环形成( Intra-hepatic collateral vessels)
-
多血管结节( Hypervascular nodules)
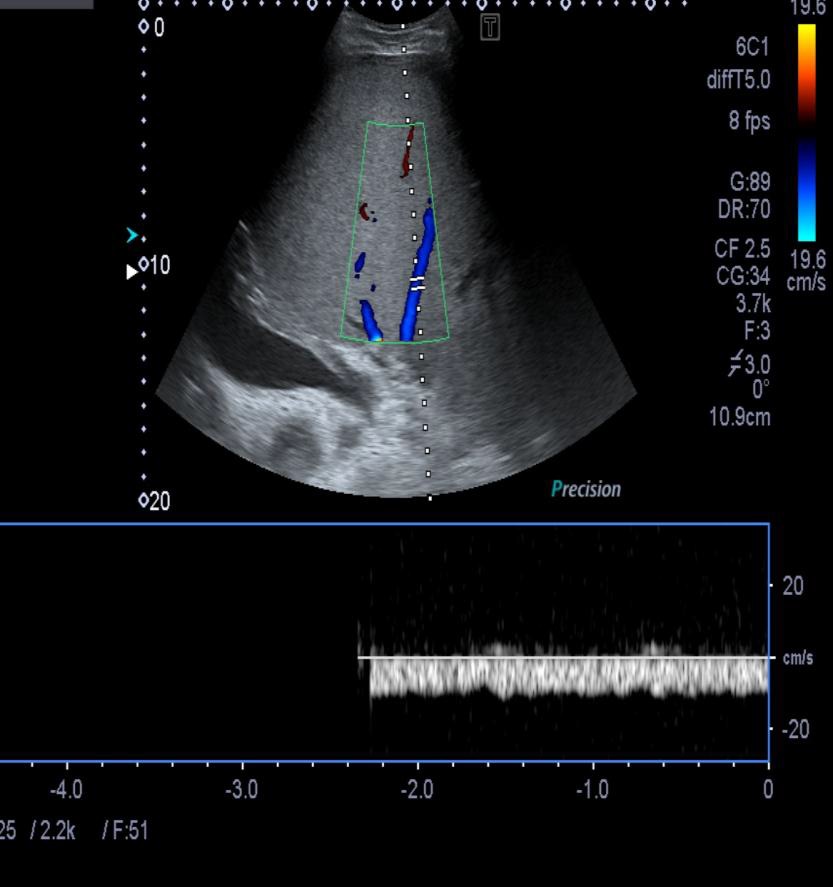
当肝静脉流出道阻塞的情况下可以显示阻塞上游静脉的扩张,侧支循环形成和没有血流信号。在急性BCS,肝静脉可以扩张,狭窄,迂曲;在慢性BCS,肝静脉有时难以识别。较少特异性的征象如脾大(78%),肝实质不均匀(76%),肝内侧支循环(73%),腹水(56%),肝外侧支循环(44%),肝尾叶肥大(67%)可以看到【1】。BCS患者肝尾叶的静脉引流会引起肝尾叶肥大(可以导致下腔静脉狭窄)而其它部分的肝脏组织相对萎缩。影像学还可以进行原发性和继发性BCS的鉴别诊断,如可以发现占位性病变或肿瘤侵入下腔静脉或肝静脉的位置。
当怀疑BCS的时候,DUS应该被认为是首选的检查方式,其敏感性为87.5%【2】。正常肝脏所有三支肝静脉都能发现。正常情况下左和中肝静脉联合独立于右肝静脉引流到下腔静脉。超声检查之后,CT的应用可以确定BCS的诊断。CT可以证实造影剂通过肝静脉失败。由于不均匀灌注,肝脏实质表现为“斑驳(mottled)”或“跳骚咬的(flea bitten)”。门静脉高压的肝外特征如脾大和腹水也可被CT所发现。但是CT可以表现为假阳性[3],而DUS发现可以是有益的。MRI的优点与CT类似,而且还可以用于发现肾脏的受累。直接静脉造影已经罕见用于直接诊断,除非需要介入治疗。
 |
-
诊断的敏感性85%,特异性90%
-
首选影像诊断技术
-
肝静脉,不可见
-
肝静脉缺乏血流
-
狭窄
-
肝静脉血栓
-
肝内侧支循环形成
-
肝段下腔静脉阻塞
-
肝尾叶肥大
|
BCS患者肝内良性多血管再生结节(Benign hypervascular regenerative nodules)较为常见,大小在0.5~4.0之间。虽然大多数在此范围内多为较小结节,但是在随访期间,它们出现时更常被注意到。而且随着时间的推移,再生结节在数量和大小都会增加。其发生被认为是肝区域内代偿肥大,这一区域的动脉血流维持原状,而有效的门静脉入血流减少。
BCS患者的良性肝结节通常是多发和多血管的【4】。>1cm 良性结节的40%通常可以发现中心疤痕,但是这一特征在小结节病变不能被发现。增强前和增强后早期的CT上,中心疤痕是低密度的。在延迟期,中心疤痕是高密度的。在MRI,中心疤痕在T1WI是低信号强度的,但在T2WI是高信号强度。BCS的这些结节的CT和MRI表现通常类似于局灶性结节样增生(focal nodular hyperplasia )。这些结节正常情况下与典型的局灶性结节样增生同样的方式吸收(take up)和保持(retain)MRI造影剂。在CT和MRI,这些结节在动脉期显示显著的增强,但并不像肝癌那样显示快速地流逝(washout)。个别的孤立性或大结节需要随访以评估随时间生长和任何变化特征。并不十分清楚是否BCS肝癌的形成是由于这些再生结节进展还是其他与肝硬化相关的癌前病变进展而来【24】。
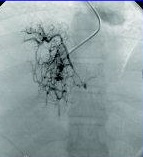
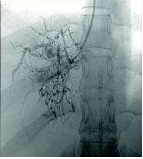
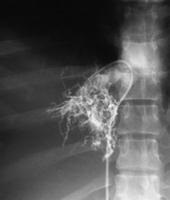
Spider web venogram
有很多的报告指出BCS与肝癌相关【5-8】。BCS经常伴有肝硬化,但肝癌发生的危险是不清楚的。一项研究显示97例病人平均随访5年11例发现肝癌;随访期间累及肝癌发生率为4%【9】。HCC与男性发生居多(72.7 vs 29.0% p =0.007),下腔静脉阻塞为(81.8 vs 4.6%)。有作者报告自己的资料认为迄今为止BCS肝癌发生率较低【10】。
可疑或增大的局灶性病变的活检,显著增高的α-FP 或可接近结节的外科切除可以确定肝癌的诊断。一项研究认为AFP>15,具有显著性意义【9,10】,并建议BCS 结节处理策略:
Management of nodules in Budd–Chiari syndrome
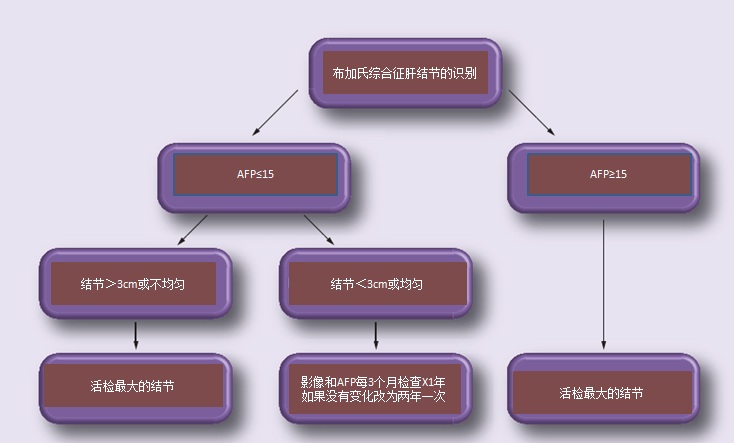
AFP: Alpha-fetoprotein; BCS: Budd–Chiari syndrome. Data taken from 【31】.
肝活检(非肝内结节活检)罕见应用于BCS患者,除非需要除外其它原因的肝脏疾病或临床高度怀疑BCS,影像学难以证实血管阻塞。与肝流出道梗阻有关典型的组织学发现是发生在小叶中心区的肝细胞缺血性缺失(ischemic loss),纤维化和充血。慢性充血可以导致肝硬化和肝实质消亡(正常结构瓦解和近似的肝和门脉通道,hepatic and portal tracts)。伴随门静脉血栓的病人,纤维化主要是门静脉周围的(periportal)【12】。同时存在门静脉血栓可导致结节样再生增生(nodular regenerative hyperplasia)*。组织学发现并不与预后相关,可能是由于阻塞和取样区域分布不均的性质【13】。
一旦确诊,应该积极开始继续检查。如果可能易发生血栓危险因素应该得到筛查(下表)。正如以前所讨论过的,多个潜在的危险因素常被发现,一个危险因素的诊断并不应中断调查另一个或几个危险因素。很多凝血因子通过肝脏合成,伴随着肝功能的不良而出现异常。
Prothrombotic conditions associated with Budd–Chiari syndrome
Prothrombotic
condition (%) |
Investigations |
Myeloproliferative
disease (49%) |
JAK2 (V617F) mutation, bone marrow biopsy |
Paroxysmal nocturnal
hemoglobinuria (19%) |
Flow cytometry of peripheral blood cells showing CD55 and
CD59 deficient clones |
|
Factor V Leiden (12%) |
Factor V Leiden mutation is more resistant to activated protein C
and therefore takes longer to switch off clotting. Clotting is
tested in the presence and absence of activated protein C to
establish if there is resistance. The factor V Leiden mutation
(R506Q) may also be identified through molecular analysis |
Prothrombin gene
mutation (3%) |
Molecular analysis for G20210A mutation |
Inherited protein C
deficiency (4%) |
Qualitative or quantitative defect in protein C. The assay is a
generally a functional one and identifies both qualitative and
quantitative deficiency |
Inherited protein S
deficiency (3%) |
Qualitative or quantitative defect in protein S. ELISA for free
protein S antigen. Functional protein S test also available |
Hyperhomocysteinemia
(22%) |
Raised serum homocysteine level |
|
Behçet’s disease (4%) |
Oral ulcers at least three times within a 1-year period along with
two out of the following four ‘hallmark’ symptoms: genital
ulcers, skin lesions, ocular inflammation and pathergy reaction
(papule >2 mm, 24–48 h or more after needle-prick) |
Antiphospholipid
syndrome (25%) |
Diagnosis of antiphospholipid syndrome requires one clinical
manifestation; arterial, venous or small vessel thrombosis, one or
more unexplained deaths of a morphologically normal fetus at or
beyond the 10th week of gestation and/or three or more
unexplained consecutive spontaneous abortions before the 10th
week of gestation, and one laboratory manifestation on two or
more occasions; anticardiolipin IgG and/or IgM and anti-β2
glycoprotein I IgG and/or IgM |
Oral contraceptives or
pregnancy (33%) |
|
|
Ulcerative colitis (8%) |
Colonoscopy if symptoms suggestive |
|
Celiac disease |
Anti-TTG and anti-EMA antibodies |
Data taken from 【14】.
因此低蛋白C,蛋白S和抗凝血酶水平的解释在BCS患者的解释是非常困难的。所有维生素K依赖凝血因子全线减少提示肝功能失不良,而单一凝血因子减少可能代表预先不足(pre-existing deficiency)有血栓家族史和使用基因检测可以协助诊断。
发现潜在的风险因素不会改变最初的治疗方案,但可能会影响长期的预后和安排家族的筛选。溃疡性结肠炎,腹腔和全身炎症疾病应除外,就像需要除外MPD一样。重要的是认识到MPD的外周血计数可能是正常的。
1. Boozari B, Bahr MJ, Kubicka S, Klempnauer J, Manns MP, Gebel M. Ultrasonography in patients with Budd–Chiari syndrome: diagnostic signs and prognostic implications. J. Hepatol. 49(4), 572–580 (2008).
2. Bolondi L, Gaiani S, Li Bassi S et al. Diagnosis of Budd–Chiari syndrome by pulsed Doppler ultrasound. Gastroenterology 100(5 Pt 1), 1324–1331 (1991).
3. Gupta S, Barter S, Phillips GW, Gibson RN, Hodgson HJ. Comparison of ultrasonography, computed tomography and 99mTc liver scan in diagnosis of Budd–Chiari syndrome. Gut 28(3), 242–247 (1987).
4. Maetani Y, Itoh K, Egawa H et al. Benign hepatic nodules in Budd–Chiari syndrome: radiologic-pathologic correlation with emphasis on the central scar. AJR. Am. J. Roentgenol. 178(4), 869–875 (2002).
5. Asrani SK, Larusso NF. Fibrolamellar hepatocellular carcinoma presenting with Budd-Chiari syndrome, right atrial thrombus and pulmonary emboli. Hepatology 55(3), 977–978 (2012).
6. Havlioglu N, Brunt EM, Bacon BR. Budd–Chiari syndrome and hepatocellular carcinoma: a case report and review of the literature. Am. J. Gastroenterol. 98(1), 201–204 (2003).
7. Takayasu K, Muramatsu Y, Moriyama N et al. Radiological study of idiopathic Budd–Chiari syndrome complicated by hepatocellular carcinoma. A report of four cases. Am. J. Gastroenterol. 89(2), 249–253 (1994).
8. Walldorf J, Tannapfel A, Holzhausen HJ et al. Rapid development of a hepatocellular carcinoma in isolated thrombosis of hepatic veins (classic Budd–Chiari syndrome): case report and review of literature. BMJ Case Rep. 2009 (2009).
9. Moucari R, Rautou PE, Cazals-Hatem D et al. Hepatocellular carcinoma in Budd–Chiari syndrome: characteristics and risk factors. Gut 57(6), 828–835 (2008).
• Good review on hepatocellular carcinoma in BCS.
10. Al-Hilou H, Olliff SP, Mangat K et al. The use of PTFE covered Transjugular Intrahepatic Portosystemic Stent Shunts (TIPSS) in Budd Chiari syndrome: long term follow up and outcomes. 62nd AASLD meeting. Hepatology 54(S1), 1238A–1238A (2011).
11 Plessier A, Rautou PE, Valla DC. Management of hepatic vascular diseases. J. Hepatol. 56(Suppl. 1), S25–S38 (2012).
• Most up-to-date review of BCS, written by highly reputed international experts.
12 Henrion J. Ischemia/reperfusion injury of the liver: pathophysiologic hypotheses and potential relevance to human hypoxic hepatitis. Acta Gastroenterol. Belg. 63(4), 336–347 (2000).
13 Tang TJ, Batts KP, de Groen PC et al. The prognostic value of histology in the assessment of patients with Budd–Chiari syndrome. J. Hepatol. 35(3), 338–343 (2001).
14. Darwish Murad S, Plessier A, Hernandez-Guerra M et al.; EN-Vie (European Network for Vascular Disorders of the Liver). Etiology, management, and outcome of the Budd–Chiari syndrome. Ann. Intern. Med. 151(3), 167–175 (2009).
• Long-term results of European-wide study with much useful data, although some marred by heterogeneity.
* 肝结节性再生性增生(Noducar regenerative hyperplasia of liver,NRHL)是一种临床少见的肝脏疾病,临床主要表现为非肝硬化性肝源性门静脉高压症,结节性再生性增生临床病理特征为肝实质出现肝细胞增生性结节而不引起纤维化及其他重要肝小叶结构改变
|