胰腺癌属于化疗(细胞毒性药物)不敏感的肿瘤。静脉5-FU单药或5-FU与MMC,异环磷酰胺以及链脲菌素联合用药一直以来显示有限抗肿瘤活性。胰腺癌姑息性全身化疗仅仅是轻度有效(marginally effective)。包括5FU在内的各种方案的肿瘤反应率小于20%,中位生存期为6个月[1]。其它单药,如丝裂霉素,顺铂,异环磷酰胺,链脲菌素以及联合化疗都没有比单剂5-FU更好的治疗,5-FU单药对于晚期胰腺癌成为不得不接受的姑息治疗方案[1]。只有吉西他滨可以显示在生命质量和存活率上优于单剂5-FU[2],目前被许多肿瘤治疗医生视为参照治疗的标准。全身化疗的效果差可能的解释由于存在多药抗药基因(multidrug resistance gene,MDR1)。膜蛋白质(P-glycoprotein)的基因密码在大多数胰腺癌细胞得到表达。作为胰腺癌细胞P-glycoprotein高水平的结果,所应用的药物从肿瘤细胞中流出增加,导致细胞内浓度降低[1,3]。Logan-Collins等人发现分子开关也在增加胰腺癌细胞对吉西他滨的抵抗[4]。
Chue 最近报告采用节律化疗治疗一例胰腺癌肝转移的病人出现5年生存率[5],给这种极恶穷凶肿瘤治疗带来一点希望,鼓励甚至是决断。尽管是个案报告,但作者希望该报告能够给与这种令人沮丧的疾病作斗争的病人带来一些鼓舞,更给治疗这种疾病的医生一些鼓励。
男,35岁 节律性化疗-胰腺转移癌5年生存
|
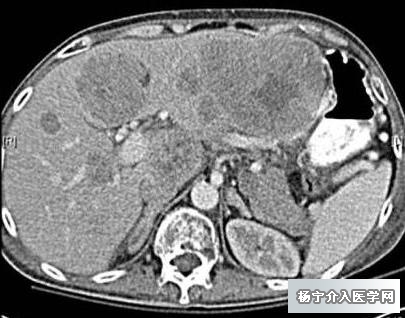
2004年CT提示肝转移癌
|
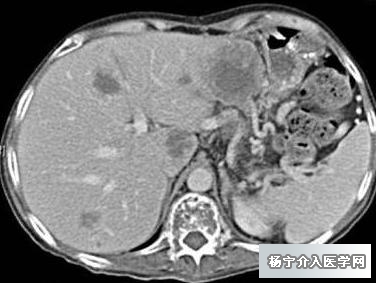
两个多月后CT显示肝转移瘤肿瘤明显缩小
|
|
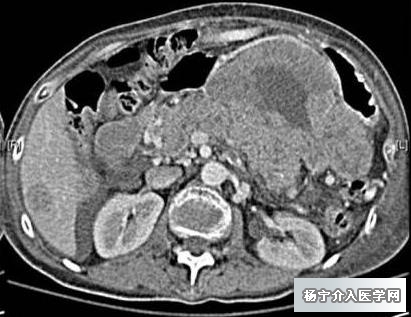
胰尾巨大占位
|

胰尾病变持续缩小
|
|
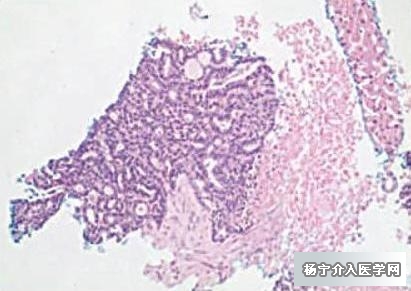
肝穿刺病理提示肝转移癌
|
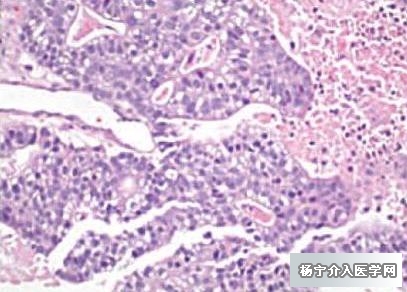
内窥镜超声下活检进一步确诊为胰腺癌
|
| 平车入院时ECOG 4分,吉西他滨治疗失败,改行节律性化疗(POLF方案):紫杉醇60mg/㎡,奥沙利铂50mg/㎡,甲酰四氢叶酸40mg,5-FU425mg/㎡。 |
间断POLF治疗5年后,ECOG评分为0分。 |
传统上的常规化疗一般选用药物的最大耐受剂量(MTD),原则是用尽大剂量和尽短的间歇,尽可能多地杀灭肿瘤细胞,避免其毒性危及患者生命。MTD化疗法在每两个周期间有2~3周的间歇以供机体恢复。MTD化疗可使大约半数肿瘤得以完全或部分退缩,但很少能治愈或显著延长癌症患者生存时间,并且常伴毒副反应,如骨髓抑制、脱发、黏膜炎及生育能力破坏。生长因子及止吐药虽能减轻这些毒性反应,但同时明显增加了医疗费用。与短期给予毒性大的MTD相对应的另外一种化疗给药方法是小剂量高频度用药,称为“节律化疗”,节律化疗不仅能降低毒性反应,而且能提高抗肿瘤效果,并且适合与靶向药物或低毒性抗癌药物合用,其与MTD比较主要优点如下[6]。
最大耐受剂量与节律化疗比较
|
|
最大耐受剂量化疗
|
节律化疗
|
|
治疗靶点
|
增殖的肿瘤细胞
|
肿瘤血管活化的内皮细胞
|
|
抗癌效果
|
易发生耐药
|
MTD耐药者仍有效
|
|
用药
|
住院,短期
|
门诊,长期
|
|
累积给药剂量
|
大
|
大大减少
|
|
延长生存期
|
一般
|
好的多
|
|
医疗费用
|
高,极高
|
较低
|
|
毒副反应
|
严重
|
很轻
|
|
止呕、注射集落刺激因子
|
常规使用
|
很少需要,无促进CPEs之虞
|
|
与抗血管生成、分子靶向药物或瘤苗合用
|
优势未能得以显现
|
提高疗效
|
一个更稳定的,有节奏的剂量给予可导致化疗副作用减少,病人有更好的耐受性。由于比较传统化疗可以增加剂量密度(dose density )和剂量强度(dose intensity)所以还提高疗效[7]。在达到化疗效果方面,剂量密度和剂量强度的重要性是众所周知的[8~10]。此外,化疗节律性实施,由于对血管内皮细胞的影响可以有抗血管生成的作用[11~14],特别是紫杉醇[15,16],或许也有5 - FU.[17,18]。抑制或逆转肿瘤血管生成在癌症治疗上的作用是非常肯定的[19~20],最近研究结果显示,每周节律性剂量给予紫杉醇在乳腺癌的新辅助治疗[21],辅助治疗[22]以及转移性病变治疗[23]的有效性将超过以前的每3周标准剂量给予的治疗,在卵巢癌,可以看到类似的好处[24]。
抗血管生成药物贝伐单抗(一种抗血管内皮生长因子单克隆抗体)的研究表明,抗血管生成药物可降低肿瘤间质压力,这可能会增加化疗进入肿瘤血管床,从而提高疗效[25,26]。因此每周紫杉醇(也有抗血管生成效果)给药可能有助于增加其他化疗药物如奥沙利铂和5氟尿嘧啶疗效。因为所需要化疗药物剂量低,获得肿瘤血管床内化疗药物相同或更高浓度,所以达到更好的抗肿瘤效果,而全身副作用少。
1. Ahlgren JD. Chemotherapy for pancreatic carcinoma. Cancer 1996;78:654-63.
2. Burris HA, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13
3. Scheithauer W. Current status of chemotherapy for pancreatic cancer: possibilities and limitations. Wien Klin Wochenschr 1994;106:704-8
4. Jocelyn Logan-Collins, Ryan M. Thomas, Peter Yu, Dawn Jaquish, Evangeline Mose, Randall French, William Stuart, Rebecca McClaine, Bruce Aronow, Robert M. Hoffman, Susan E. Waltz. Andrew M. Lowy. Silencing of RON Receptor Signaling Promotes Apoptosis and Gemcitabine Sensitivity in Pancreatic Cancers Cancer Research 70, 1130, February 1, 2010.
5. Ben M. Chue, MD Five-Year Survival of Metastatic Pancreatic Carcinoma: A Study of Courage and Hope Gastrointest Cancer Res. 2009 Sep–Oct; 3(5): 208–211
6. 李醒亚 肿瘤节律化疗研究进展 第十一届全国临床肿瘤学大会暨2008年CSCO学术年会
7. Seidman A. Will weekly work?Seems to be so. J Clin Oncol. 2005;23:5873–5874
8. Frei E, 3rd, Canellos GP. Dose: a critical factor in cancer chemotherapy. Am J Med. 1980;69:585–594.
9. Norton L. Evolving concepts in the systemic drug therapy of breast cancer. Semin Oncol. 1997;24(suppl 10):S10-3–S10-10.
10. Kwak LW, Halpern J, Olshen RA, et al. Prognostic significance of actual dose intensity in diffuse large-cell lymphoma: results of a tree-structured survival analysis. J Clin Oncol. 1990;8:963–977.
11. Phillips C. A new “target” for chemotherapy? Natl Cancer Inst Cancer Bull 3263–5.2006. Available at http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_062706/page4.
12. Bertolini F, Saki P, Mancuso P, et al. Maximum tolerable dose and low dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res. 2003;65:4342–4346.
13. Kerbel RS, Kamen B. The antiangiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–436.
14. Shaked Y, Emmenegger U, Cervi D, et al. Optimal biologic dose of metronomic chemotherapy regimens is associated with maximum antiangiogenic activity. Blood. 2005;106:3058–3061.
15. Lau D, Guo L, Gandara D, et al. Is inhibition of cancer angiogenesis and growth by paclitaxel schedule-dependent? Anti-Cancer Drugs. 2004;15:871–875.
16. Belotti D, Vergani V, Drudis T, et al. The microtubule-affecting drug paclitaxel has antiangiogenic activity. Clin Cancer Res. 1996;2:1843–1849.
17. Dastur YK, Dasgupta S, Chitele A, et al. The role of initial 5-fluorouracil trabeculectomy in primary glaucoma. J Postgrad Med. 1994;10:197–201.
18. Chaudhry IA, Pasha MA, O’Connor DJ, et al. Randomized, controlled study of low-dose 5-fluorouracil in primary trabeculectomy. Am J Ophthalmol. 2000;130:700–703.
19. Bergers G, Benjamin L. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410
20. Kerbel R. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049.
21. Green MC, Buzdar A, Smith T, et al. Weekly paclitaxel improves pathological complete remission in operable breast cancer when compared with paclitaxel once every three weeks. J Clin Oncol. 2005;23:5983–5992.
22. Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671.
23. Seidman A, Berry D, Cirrincione C, et al. Randomized phase III trial of weekly compared with every three weeks paclitaxel for metastatic breast cancer with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B Protocol 9840. J Clin Oncol. 2008;26:1642–1649.
24. Isonishi S, Yasuda M, Takahashi F, et al. Randomized phase III trial of conventional paclitaxel and carboplatin (c-TC) versus dose dense weekly paclitaxel and carboplatin (dd-TC) in women with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer: Japanese Gynecologic Oncology. 2008 ASCO Annual Meeting Proceedings. J Clin Oncol. 2008;26(May 20 suppl) (abstr 5506).
25. Willett CG, Boucher Y, Munn LL, et al. Direct evidence that the VEGF-specific antibody bevacizumab has anti-vascular effects in human rectal cancer. Nat Med. 2004;10:145–147.
26. Jain RK. Taming vessels to treat cancer. Sci Am. 2008;298:56–63. | 






